Research Strategic Plan

In 2019, the Department of Medicine invested considerable effort and resources to devising a strategic plan that will provide a roadmap for our research mission today and into the future.
This work was guided by a Research Planning Committee that convened throughout the first half of 2019, reviewing the current state of research in the Department, generating recommendations for strengthening our research efforts, and developing the following plan. Many of our faculty and research administrators participated and contributed ideas as part of this process—through interviews, a survey, and robust discussions at the 2019 Research Retreat.
The result of this combined effort is the clear, direct, ambitious, and ultimately achievable research strategic plan that follows.
We identified five strategies for achieving our vision.
We will foster the success of our current faculty by enhancing our faculty development, mentoring, and funding programs while also strengthening the pipeline of the next generation of outstanding investigators in Medicine.
Lead: Andrew Alspaugh, MD
Initiatives:
- Strengthen faculty career development programs (Xunrong Luo, Matthew Crowley)
- Build a diverse and inclusive Department of Medicine (Laura Svetkey, Julius Wilder)
- Foster a culture of outstanding mentorship in the Department (Alspaugh, Cathleen Colon-Emeric)
- Expand physician-scientist recruitment and programmatic support (Rodger Liddle, Matt Hirschey)
- Launch a Department partnership hires program (Xunrong Luo, Chris Holley)
- Expand cadre of independent PhD investigators (Scott Palmer, Amy Porter-Tacoronte)
We will enhance our partnerships with other departments, centers, institutes, schools, and programs across Duke University.
Lead: David Simel, MD, vice chair for veterans affairs
- Duke Clinical Research Institute
- Duke Cancer Institute
- Durham VA Medical Center
- Duke Molecular Physiology Institute
- Pratt School of Engineering and MEDx
- Duke Human Vaccine Institute
- Duke Global Health Institute
- Center for Applied Genomics and Precision Medicine
We will solidify a leadership position in data science by leveraging the clinical disease expertise of our faculty; building our data assets; and improving our data collection, storage and analytics resources.
Lead: Chetan Patel, MD, vice chair for clinical affairs
- Cultivate DOM data assets into open science platform
- Augment biostatistics & bioinformatics resources
- Create new leadership role for data science
- Implement learning health units
- Continue implementation of Science Culture and Accountability Plan
We will foster a community and culture of rich scientific investigation by making research easier while achieving the highest levels of research integrity.
Lead: Erica Malkasian
- Provide outstanding grants and administrative support to investigators
- Position Duke as a leader in site-based research
- Develop next-generation biorepository capabilities
- Catalyze innovation and entrepreneurship
- Expand international research efforts
We will invest in emerging research content and method areas that leverage our strengths and address important unmet patient-centered medical needs.
Lead: Heather Whitson, MD
Cross-cutting themes:
- Immunology, inflammation & fibrosis
- Aging, resilience & pain
- Energy, obesity & metabolic disease
- Precision medicine
- Population health & disparities research
To learn more about our research strategies and initiatives, contact
- Scott Palmer, MD, MHS, Vice Chair for Research
- Saini Pillai, MBA, Senior Program Coordinator, Research

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Research Initiatives
- Research at NINR
- Research Supported by NINR
- RESEARCH LENSES
- Health Equity
- Social Determinants of Health
- Population and Community Health
- Prevention and Health Promotion
- Systems and Models of Care
- Funding Opportunities
- Small Business Funding
- Grant Applicant Resources
- Training Grants
- Featured Research
- Strategic Plan
- Budget and Legislation
- Connect With Us
- Jobs at NINR


The National Institute of Nursing Research 2022-2026 Strategic Plan
The National Institute of Nursing Research 2022-2026 Strategic Plan outlines the Institute's vision for supporting science that advances our mission: to lead nursing research to solve pressing health challenges and inform practice and policy-optimizing health and advancing health equity into the future.
Nurses are crucial to solving the Nation's most pressing and persistent health challenges. As both the largest health profession and the largest of all professions in the Nation, nurses are the backbone of our health systems in the United States and have ranked as the most trusted profession for 20 years in a row. Nurses interact with individuals and families more closely than other health professionals in the many clinical, community, and policy settings in which they work; thus, they have a deep understanding of the personal and societal factors that lead to health among some, and illness among others. NINR believes that nursing research is the key to unlocking the power and potential of nursing by leveraging these strengths and unique knowledge and perspectives inherent to the discipline to the benefit of all people.
Nursing's earliest pioneers recognized that health must be considered within the context of people's lives and living conditions. Florence Nightingale was one of the first to recognize and address the connection between health and environmental elements, such as ventilation and warming, clean air and water, noise pollution, and provision of light. Lillian Wald saw nurses as working at the intersection of medicine and society to care for individuals, families, and communities in the context of social, economic, and industrial conditions. Nurses continue to build on this rich history through nurse-led efforts to address intensifying inequities and social determinants of health.
This strategic plan describes how NINR will support scientific programs, training, and policies that both move the nursing research field forward and maximize the impact of our science through good stewardship of public funds. The plan includes our Director's overview, research framework, stewardship plan, development process, and frequently asked questions. The changes from prior plans are intentional and were made in recognition that our science needs to continue to innovate. As a living document, this plan will allow NINR to respond nimbly to evolving and emerging health issues facing our Nation.

This fact sheet provides an overview of the National Institute of Nursing Research's (NINR) 2022-2026 strategic plan, which includes NINR's mission, research lenses for investigating health-related questions, guiding principles for prioritizing research, and a research framework for achieving NINR's mission.

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

2023-2027 NIDCD Strategic Plan

Advancing the Science of Communication to Improve Lives
Overview and Introduction
Director’s message, nidcd mission, nidcd vision, nidcd overview, nidcd statutory authority, establishment, other statutory authority, nidcd organizational chart, serving as an efficient and effective steward of public resources, setting priorities, excel as a federal science agency by managing for results, nih-wide crosscutting priorities, seek innovation through partnerships, strengthen research training and career development, reinforce a culture of scientific workforce diversity, equity, inclusion, and accessibility (deia), participate in international research to improve global health, advance research to improve women’s health, reduce health disparities, future directions in nidcd scientific program areas, theme 1: capitalize on advances in basic research to enhance our understanding of normal function and disordered processes., theme 2: develop and improve model systems to inform research., theme 3: promote a precision medicine approach to prevention, diagnosis, and treatment., theme 4: translate and implement scientific advances into standard clinical care., theme 5: facilitate use of and best practices in biomedical data science., theme 6: harness advanced technology to improve prevention, diagnosis, and treatment., measuring progress, appendix a: nidcd strategic planning process, pre-planning, crowdsourcing new ideas.
- Drafting and Publishing Strategic Plan
Appendix B: Liaisons from the National Deafness and Other Communication Disorders (NDCD) Advisory Council
Appendix c: nidcd internal strategic plan working group.
I’m pleased to share the 2023–2027 National Institute on Deafness and Other Communication Disorders (NIDCD) Strategic Plan: Advancing the Science of Communication to Improve Lives. NIDCD’s mission is to improve the lives of the millions of people with hearing loss and communication disorders, spanning functions of hearing, balance, taste, smell, voice, speech, and language. This plan contains ambitious, achievable goals for the science in our mission areas—goals that will further our scientific understanding of basic biological systems, human disease mechanisms, and promising treatments.
Thank you to everyone who submitted comments on the draft themes and goals for this new plan. I appreciate the valuable input provided to us as we developed and finalized a plan to guide us for the next five years.
Over the past 34 years, researchers supported by NIDCD have made seminal advances leading to increasingly effective, evidence-based treatments for diseases and disorders that affect an ever-growing segment of the population. These remarkable advances in science, technology, and computing create unprecedented opportunities for knowledge discovery, clinical translation, and public health. NIDCD’s vision statement reflects our hope to leverage these opportunities: Advancing the science of communication to improve lives.
Our 2023–2027 Strategic Plan was developed through a collaboration between NIDCD staff, the scientific community, members of the public, advocacy groups, and professional organizations. NIDCD began development of the plan by examining both our currently funded research project portfolio and available assessments of public health need and disease burden in our mission areas. We then solicited input from the scientific community, requesting bold ideas that would lead to breakthrough advances in our scientific mission areas. These ideas were further explored both in virtual meetings with members of the scientific community and by NIDCD scientific staff, leading to the six major themes and accompanying aspirational goals of the plan. Additional input was obtained from our public constituencies during the public comment period. These comments were carefully considered, and changes to the plan were made where appropriate. The process of refining the plan depended upon the thoughtful input of NIDCD’s collaborators and helped us to identify areas of unmet research needs and opportunity, including public health challenges that fall within our mission and vision. NIDCD will use this plan to guide our research investments. Our goal is to support innovative, crosscutting, multidisciplinary research so that all Americans can benefit from scientific discovery that will inform effective and accessible prevention strategies and treatments for deafness and communication disorders.
Thank you for your interest in NIDCD's scientific research.
Debara L. Tucci, M.D., M.S., M.B.A. Director National Institute on Deafness and Other Communication Disorders
The NIDCD mission is to conduct and support research and research training in the normal and disordered processes of hearing, balance, taste, smell, voice, speech, and language.
Advancing the science of communication to improve lives.
NIDCD conducts and supports research and research training related to disease prevention and health promotion; addresses special biomedical and behavioral problems experienced by people who have communication impairments or disorders; supports research evaluating approaches to the identification and treatment of communication disorders and patient outcomes; and supports efforts to create devices that substitute for lost and impaired sensory and communication function.
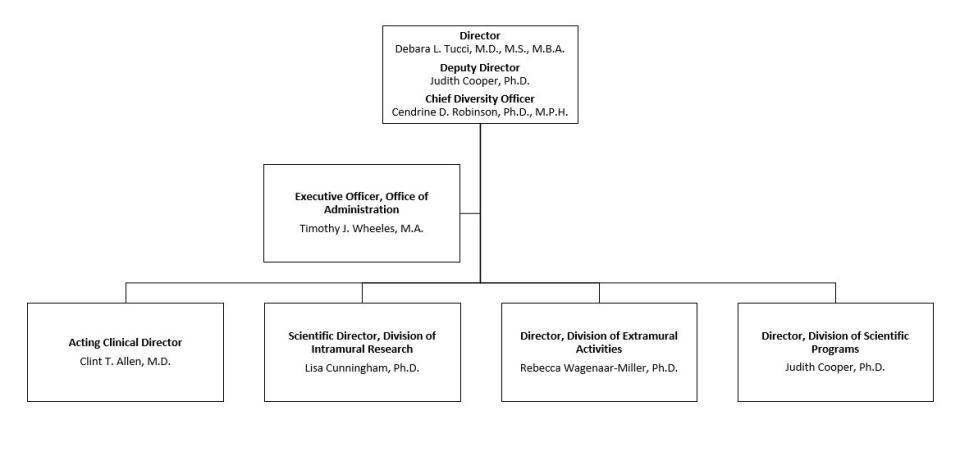
NIDCD accomplishes its research mission through three divisions: the Division of Intramural Research (DIR), the Division of Scientific Programs (DSP), and the Division of Extramural Activities (DEA). DIR conducts research and related support activities in laboratories and clinics housed at National Institutes of Health (NIH). DSP and DEA manage complementary aspects of NIDCD’s Extramural Research Program, a program of research grants, career development awards, individual and institutional research training awards, center grants, and contracts to public and private research institutions and organizations throughout the United States and abroad.
NIDCD manages a broad range of projects in both basic and clinical research. NIDCD research is organized into three program areas: hearing and balance; taste and smell; and voice, speech, and language. The three program areas seek to answer fundamental scientific questions about normal function and disorders and to identify patient-oriented scientific discoveries for preventing, screening, diagnosing, and treating disorders of human communication.
Hearing and Balance Program The NIDCD Hearing Program and Balance Program encompass over half of NIDCD’s research portfolio. To study normal and disordered functions of the auditory and vestibular systems, NIDCD employs a wide range of research approaches such as molecular genetics, cellular biology, animal models, biomedical imaging, nanotechnology, psychoacoustics, and structural and functional biology. NIDCD supports research that will lead to improved treatments for, and prevention of, hearing and balance disorders.
Imagine the Future : People with vestibular nerve tumors get effective treatments that spare hearing, balance, and facial nerve function without surgery.
Taste and Smell Program The NIDCD Taste and Smell Program supports studies of the chemical senses known as taste, smell, and chemesthesis (chemically provoked irritation) to enhance our understanding of how individuals gather information about their environment and how human chemosensory disorders can be diagnosed and treated. NIDCD-supported research on molecular and cellular biology, animal models, biophysics, and biochemistry of the olfactory and gustatory systems is paving the way for improved diagnosis, prevention, and treatment of chemosensory disorders.
Voice, Speech, and Language Program The NIDCD Voice, Speech, and Language Program uses a wide range of research approaches to develop effective diagnostic and intervention strategies for people with communication impairments. Research in voice and speech determines the nature, causes, treatment, and prevention of disorders of motor speech production throughout the lifespan. Language research includes the exploration of the genetic bases of child speech and language disorders, as well as characterizing the linguistic and cognitive deficits in children and adults with language disorders.
Imagine the Future : Health care providers use brain imaging to help identify which children will develop persistent speech delay or stuttering and begin transformative therapy early.
42 U.S.C. § 285m: The general purpose of the National Institute on Deafness and Other Communication Disorders (hereafter referred to in this subpart as the “Institute”) is the conduct and support of research and training, the dissemination of health information, and other programs with respect to disorders of hearing and other communication processes, including diseases affecting hearing, balance, voice, speech, language, taste, and smell.
President Ronald Reagan signed P.L. 100-553 into law on October 28, 1988, establishing NIDCD as a new institute within NIH.
Until that time, research on communication sciences had been supported by NIH through the National Institute of Neurological and Communicative Disorders and Stroke (now the National Institute of Neurological Disorders and Stroke). Individuals with hearing loss, researchers, clinicians, and professional societies worked together to advocate to Congress to authorize a new institute at NIH. Representative Claude Pepper (D-FL) and Senator Tom Harkin (D-IA) introduced the National Deafness and Other Communications Disorders Act of 1988 (S. 1727), which quickly passed in Congress and was signed into law, establishing a new NIH institute dedicated to research and research training in hearing, balance, taste, smell, voice, speech, and language.
Another provision in the Public Health Services Act (42 U.S.C. § 280g-1) directs the NIH Director, acting through the NIDCD Director, to continue a program of research and development on the efficacy of new screening techniques and technology, including clinical studies of screening methods, studies on efficacy of intervention, and related research for early detection, diagnosis, and treatment for newborns, infants, and young children who are deaf or hard-of-hearing.
[ Text version ]
The first step in prioritization is to identify the most promising research. NIDCD sets research priorities by identifying areas of scientific opportunity that will address the greatest public health needs. NIDCD encourages investigator-initiated applications in basic, translational, and clinical research that help achieve the NIDCD mission. In particular, the Institute encourages investigators to submit applications for research projects that directly address themes and strategic goals within NIDCD's strategic plan. NIDCD will, however, remain flexible to respond to emerging scientific or clinical challenges and new opportunities.
NIDCD uses the NIH system of peer review to evaluate meritorious research grant applications. A panel of scientific experts from outside of NIH, with both broad and specialized expertise, review the applications for overall scientific and technical merit. To learn more about the NIH peer review process, see https://grants.nih.gov/grants/peer-review.htm .
NIDCD promotes the plan to the research community to increase awareness of Institute priorities. The plan creates a unified vision for the future with researchers, the public, and policymakers. To develop the 2023–2027 Strategic Plan, NIDCD solicited input from scientific experts, the National Deafness and Other Communication Disorders (NDCD) Advisory Council, NIDCD staff, and the public. See Appendix A for more details on the strategic planning process.
NIDCD is a public science agency supported by federal funds. As part of NIH, NIDCD is obligated to base its funding decisions on the strength of the proposed science and to make its decision-making process transparent. NIDCD upholds its accountability to the American public by managing its scientific endeavors to achieve results that improve the lives of individuals who are deaf, hard-of-hearing, or presenting with communication disorders. The accountability process includes reporting, as required by the Government Performance and Results Act (GPRA); an administrative strategic plan that ensures effective, responsive management and operations; and agile plans to mitigate the risks involved in fulfilling the NIDCD mission.
GPRA , a public law passed by Congress in 1993 (Public Law 103-62) to improve stewardship in the Federal Government, links resources and management decisions with program performance. To comply with GPRA, NIH develops an annual plan proposing goals that provide a representative sample of NIH’s activities for each year; describes how these goals will be met; and provides evidence to support any claims for successful achievement of the goals. Each Institute and Center at NIH participates in the GPRA reporting process, including NIDCD.
Internally, NIDCD also effectively manages its public funds for results by developing and using an NIDCD Administrative Strategic Plan. Every three years, NIDCD staff identify administrative challenges at the Institute and develop an NIDCD Administrative Strategic Plan to address these challenges. The Administrative Strategic Plan helps NIDCD manage its administrative services in support of the NIDCD’s mission by:
- Modeling innovative management approaches and sharing best practices within and between NIDCD offices
- Improving employee quality of life and job satisfaction by implementing clear, consistent administrative service practices
- Providing better decision-making and transparency by setting and tracking goals
Annually, NIDCD conducts a risk inventory assessment to support the NIH Director’s Financial Integrity Act attestation and develops a risk management plan to ensure that public funds are invested and administered in compliance with applicable laws, regulations, and policies. The plan examines NIDCD’s activities and assesses risks, establishes methods for control of those risks, monitors adherence to the risk-reduction methods, and mitigates risks involved in administering the NIDCD mission. NIDCD’s assessment tries to minimize the risk of failure in all NIDCD activities, and it is submitted each year as part of the overall NIH Risk Management Program .
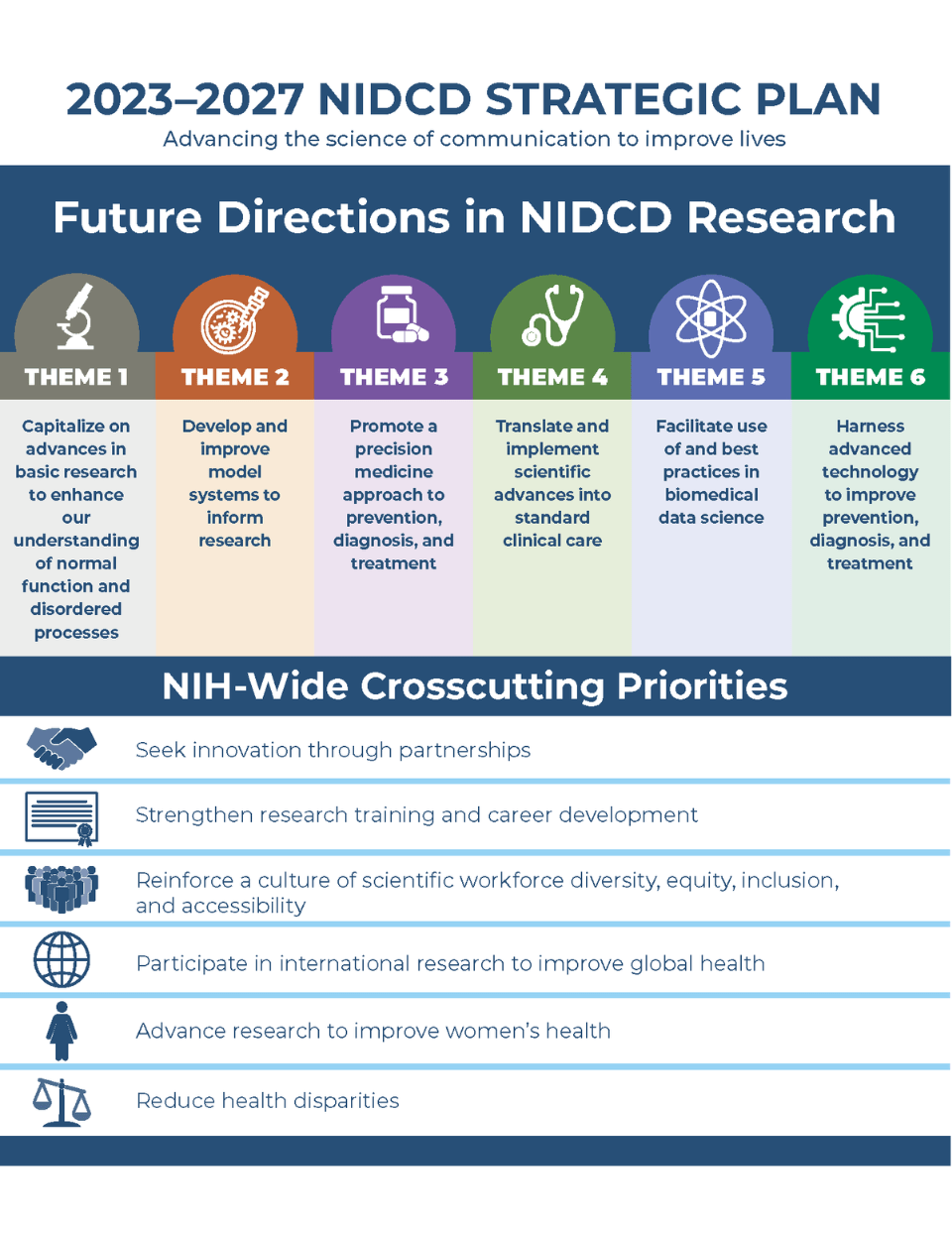
Many scientific challenges and opportunities are not unique to any one theme in this plan. To emphasize this, NIDCD has identified six key crosscutting priorities that span all aspects of NIDCD’s mission. These priorities are:
- Seek innovation through partnerships
- Strengthen research training and career development
- Reinforce a culture of scientific workforce diversity, equity, inclusion, and accessibility (DEIA)
- Participate in international research to improve global health
- Advance research to improve women’s health
- Reduce health disparities
[ Download PDF | Text version ]
NIDCD continues to partner with other NIH Institutes and Centers in related research areas to leverage what they learn to advance our own mission. Examples include the BRAIN Initiative® , All of Us Research Program , and NIH-wide initiatives in the areas of Alzheimer’s disease and related dementias, and Down syndrome . These partnerships improve our understanding of communication disorders and encourage innovation. Read more about NIH-wide and trans-agency efforts in the “Leveraging Partnerships” section of the NIH-Wide Strategic Plan .
NIDCD recognizes the importance of research training and career development opportunities to ensure a productive, diverse, creative, and innovative cadre of qualified scientists in basic, clinical, and translational research in the Institute’s mission areas. NIDCD is continuously adapting its research training and career development efforts to help new scientists establish careers in our mission areas, encourage clinicians to pursue opportunities in translational research, and use shared research resources.
The field of human communication sciences greatly benefits from interdisciplinary research teams to bridge the gap between laboratory research and patient care. Such teams provide clinicians with a deeper understanding of the latest research discoveries, facilitating the introduction of new diagnostic and treatment approaches into the clinic. Likewise, these collaborations provide basic researchers with a more thorough understanding of patients’ needs, challenges, and opportunities. The creation of such interdisciplinary teams—in which scientists with varied perspectives are cross-trained by one another—could spark new ways to better prevent, detect, and treat communication and chemosensory disorders by identifying new directions for scientific discovery, conducting hypothesis-driven clinical trials, assessing new diagnostic tools and interventions, and improving public health and well-being.
Research studies indicate that diverse teams working together and capitalizing on innovative ideas and distinct perspectives outperform homogeneous teams. NIDCD recognizes the critical need to address barriers that interfere with the scientific aspirations of individuals from diverse backgrounds , including women and minorities, individuals with disabilities, and individuals from rural areas and other underserved populations. NIDCD is working to increase the number of individuals from diverse backgrounds in the research pipeline in our scientific mission areas, for both the extramural and intramural programs .
NIDCD’s ongoing extramural DEIA efforts include:
- Funding opportunities to diversify our training and mentoring pipelines
- Funding opportunities to promote workforce diversity among new investigators
- How to Apply for a Grant, Research Training, or Career Development Funding
- Examples of successful grant applications to guide prospective grantees
- A webpage describing extramural research workforce diversity programs, including the NIDCD Diversity Scholars Program
- Broadening distribution of an e-newsletter, which highlights funding opportunities, upcoming webinars, updates from the NDCD Advisory Council, and other NIH resources, to recent grant applicants, as well as applicants located at Historically Black Colleges and Universities (HBCUs) and Minority-Serving Institutions (MSIs)
- Increased NIDCD participation at conferences that reach a broader audience of professionals and trainees to share information about application processes and funding opportunities
NIDCD’s ongoing intramural DEIA efforts include:
- Development and use of an NIDCD Intramural Recruitment Guide for all recruitments in the Institute
- NIDCD internal recruiting committee identification of a diverse list of highly qualified applicants to the NIH Postbaccalaureate Program for consideration by NIDCD Intramural Investigators
NIDCD created the position of Chief Diversity Officer and, in 2022, hired the first person to serve in this role . This member of the NIDCD leadership team will help direct our efforts to develop and retain a diverse extramural and intramural workforce, engage more effectively with underrepresented populations in clinical research in our mission areas, and ensure that the NIDCD workplace is diverse, respectful, and inclusive. NIDCD recognizes that our ability to remain global leaders in scientific discovery and innovation depends upon a pool of highly talented scientists from diverse backgrounds.
Imagine the Future : Regardless of background, people of all ages who want to engage in and contribute to science will have access to education, training, and career opportunities.
NIDCD aims to reduce the burden of hearing loss and communication disorders in the United States and around the globe. The Institute supports and facilitates global health research at both domestic and foreign institutions. NIDCD efforts include support of collaborations between U.S. and international researchers at institutions worldwide, expanding the scientific workforce, and improving access to the world’s collective scientific talent and expertise. NIDCD also aims to train the next generation of scientists to address global health concerns in deafness and communication disorders.
By supporting international research, NIDCD can accelerate the discovery of causes and interventions for deafness and communication disorders. For example, NIDCD-supported international collaborations based in the NIDCD intramural program facilitated the collection of genetic data from families around the world who are affected by deafness and stuttering. This research uncovered gene variations associated with these conditions and provided additional insights into their causes and potential targeted treatments.
Learn more about current international initiatives that are related to NIDCD’s mission.
Imagine the Future : Children with hearing loss, anywhere in the world, can access the most effective treatment options – including biologic therapy or advanced technologies – to reach their full potential.
Recognizing the importance of both advancing research related to women’s health and encouraging women to pursue biomedical research careers, NIDCD is working to promote women’s health research, expand the understanding of sex differences, cultivate and retain women in biomedical careers, and ensure the inclusion of women in research. NIDCD reports our efforts to include women in health research and clinical trials in our biennial update to help evaluate progress on the goals described in the Trans-NIH Strategic Plan for Women's Health Research .
NIDCD recognizes that medical advances and technology improvements have not improved the health of all Americans equally. Health disparities, reflected in disparate access to quality health care interventions, have a disproportionately high impact on racial and ethnic minority populations, individuals of less privileged socioeconomic status, underserved rural residents, and sexual and gender minorities. NIDCD participates in and stands behind the NIH Minority Health and Health Disparities (MHHD) Strategic Plan 2021–2025 . NIDCD is taking action to achieve the MHHD Strategic Plan goal to advance the scientific understanding of health disparities. For example, NIDCD aims to define the rates of hearing impairment by race and ethnicity by 2030. NIDCD’s research opportunities to promote diversity (described above) also support the MHHD Strategic Plan goals aimed at broadening the cadre of individuals trained to conduct health disparities research.
Imagine the Future : Clinicians utilize research data on sex and gender to improve diagnosis and treatment of communication disorders.
NIDCD looks to the scientific community to identify the most promising research and will continue to support investigator-initiated projects. Our collaborators identified the themes and goals during our strategic planning process as areas of opportunity for the Institute. Accordingly, these areas are of high priority for the Institute over the next five years. Within the themes and goals outlined below, we use the terms “communication” and “sensory” in relation to all of NIDCD’s seven mission areas: hearing, balance, taste, smell, voice, speech, and language.
Basic research is foundational for all NIDCD mission areas, and scientific progress requires interdisciplinary approaches to develop new technologies, improve methods of data analysis, and provide insight on fundamental disease pathways. NIDCD seeks to leverage new technologies and recent discoveries to better understand normal and disordered function of cells, circuits, tissues, whole organs, and systems; and individual or social group behaviors that play important roles in human communication. By defining what is normal in both humans and animal models, we can better understand mechanisms of disease. Increasing our knowledge of the mechanisms of diseases, disorders, and dysfunctions that impair human communication and health can lead to fundamental advances and technological developments that inform translation to clinical care. NIDCD encourages basic research collaborations that span the traditional and emerging disciplines of life, physical, engineering, computer, behavioral, and social sciences.
Imagine the Future : People with recent exposure to potentially damaging noise may take a pill that prevents permanent loss of hearing.
Goal 1: Identify and characterize different cell populations in both peripheral and central regions.
Hearing, balance, taste, and smell depend upon highly specialized cells to detect and process sensory information. Understanding the cell types in sensory organs and the peripheral and central nervous systems is critical to the development of fundamental knowledge to guide future treatments. NIDCD encourages investigators to identify and characterize the molecular, cellular, anatomical, and functional properties of cells that are important for communication, and to discover how normal function can be disrupted in normal aging, diseases, and disorders in both model organisms and human tissue, across the lifespan.
Goal 2: Identify and characterize neural circuits involved in sensory processing.
Cutting-edge technological methods advanced by the NIH Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative allowed for the development of high-speed functional microscopy that enables scientists to image extremely rapid, physiological changes at the single-cell level and across the brain in small animal model systems. Next-generation electrode arrays allow simultaneous recordings from hundreds to thousands of neurons over time, providing unprecedented temporal resolution of neural circuits to produce a dynamic picture of brain function in larger animals. The Institute recognizes the importance of efforts to quantify behaviors and combine them with simultaneous recordings of brain activity in humans. NIDCD seeks multidisciplinary and innovative projects to identify and characterize the neural circuits that play a role in sensory processing for communication. NIDCD encourages research to:
- Develop a dynamic map of brain circuits involved in specific sensory pathways
- Examine the neural circuits and computations that underlie multisensory interactions in communication processing
- Determine the conservation of circuits in a sensory system across species
- Identify the changes to circuits when compromised by sensory and communication deficits
Imagine the Future : People with vocal fold injury can regenerate the cells needed to restore function.
Goal 3: Facilitate the utilization of biopsied and postmortem human tissue to advance research.
Working toward the goal of translating basic research to clinical research, studies using animal models may require validation in human tissue. In order to provide a critical link from animal studies to human tissue and eventually to clinical practice, NIDCD seeks to improve current techniques for the processing and use of donated human temporal bones, brains, and other sensory tissues. NIDCD encourages the incorporation of human tissue studies to complement current work using animal models for normal and disordered communication. New and/or improved methods are needed for characterization and analysis of human tissue, such as immunohistochemistry; multi-modal, multi-omic sequencing and analyses; and other emerging techniques. NIDCD encourages researchers to consider how to best use new imaging techniques and tissue preservation methods to study human temporal bones, brains, or other sensory tissues with a goal toward improved understanding of human communication disorders, ultimately, in vivo .
Goal 4: Define interactions between immune-mediated networks and the influence of inflammation on normal and disordered function.
The immune system protects the body from foreign substances, such as potentially infectious agents and toxins, and responds to injury and repair of damaged tissue. The role of the immune system in communication disorders is not well understood. Because immune cells can contribute to both tissue injury and tissue repair, the signals that mediate these responses must be better characterized and defined in sensory systems. NIDCD recognizes the interplay between the nervous system, the endocrine system, and the immune system, and the need to better understand these processes to improve our ability to prevent and treat communication disorders. Resolving this knowledge gap is critical for the prevention and treatment of communication dysfunction. It is important to define the immune cell types and signaling mechanisms that contribute to communication function and dysfunction. NIDCD encourages new approaches to modulating immune responses to viruses (including COVID-19), bacteria, and/or injury that impacts sensory and communication function. The goal is to promote tissue repair and regeneration while reducing immune responses that damage sensitive cells, tissues, and organs needed for effective human communication.
NIDCD sees a continued critical need for the development of relevant model systems to study normal and pathologic conditions in human communication. The Institute encourages the development of new pre-clinical, in vivo , in vitro , and in silico model systems, as well as the improvement of current models to inform research and future clinical translation, leading to effective treatments.
Imagine the Future : Researchers use pre-clinical models to identify drug targets to treat stuttering in children and adults.
Goal 1: Develop robust in vivo and pre-clinical models to study normal and disordered function.
NIDCD seeks to support research on in vivo models to deepen our understanding of the mechanisms underlying normal and disordered function of the systems involved in human communication. By defining what is normal in animal models and humans, researchers can better understand underlying causes of communication disorders. In addition, optimization of novel therapeutics is usually dependent upon pre-clinical evaluation in animal models or ex vivo model systems. Such models can help to inform the decision to advance a therapeutic candidate to clinical testing and can provide valuable data for clinical study design. NIDCD encourages development of new pre-clinical models to provide an improved translational toolkit that will better predict the efficacy and safety of new therapeutic strategies for treating communication disorders.
Goal 2: Develop in vitro models to accelerate basic studies and high throughput screening.
Cell-based assays and in vitro models can expedite translation of basic research discoveries into more specific disease models. Such models may include new organ system platforms (i.e., “organs on chips”), or bioengineered platforms that can generate 3-dimensional models of the organ systems (i.e., organoids) from human cell sources. These models reproduce critical molecular, anatomical, mechanical, and physiological features that mediate tissue and organ function, allowing efficient monitoring and interrogation of normal and disordered processes as well as the testing of potential therapeutics. This type of in vitro model system can bridge the gap between pre-clinical testing in animals and human clinical trials, thus improving safety and efficacy of new treatments in humans while also potentially reducing the cost and time needed for development of therapies. NIDCD encourages the continued development of cell-based assays and other in vitro models for high-throughput or high-content studies, as well as disease-specific research.
Imagine the Future : A ‘tissue chip’ containing miniature versions of taste buds or olfactory epithelium allows researchers to screen for and accelerate development of safe and effective therapies for chemosensory loss.
Goal 3: Develop in silico (computer) models to enable insights into normal and disordered function.
In silico modeling uses computing power to model complex biological processes. It combines the advantages of both in vivo and in vitro experimentation, but without being subject to the ethical considerations and lack of control associated with in vivo experiments and without the restrictive parameters associated with in vitro experiments. In silico models allow the researcher to run multiple, repeated simulations of biological, behavioral, and/or social processes while tightly controlling for variables to predict how systems perform. The development of models and the use of simulation are powerful adjuncts to improving the predictive value of collected data.
The establishment, maintenance, manipulation, and/or characterization of in silico models provides the resources and components for future development of new platforms to model pathways and outcomes, including the selection of appropriate in vivo and in vitro models for an organ system. NIDCD encourages research in experimental neuroscience and in physical, computer, mathematical, and engineering sciences to develop in silico models that are useful for studying normal or disordered functions of communication and to integrate such models into research project design.
Precision medicine considers individual differences in genes, behavioral phenomena, environments, and lifestyles in the clinical decision-making process. A significant goal of precision medicine is, at the individual level, to deliver the right intervention at the right dose at the right time, and to integrate this approach broadly when developing novel therapies and interventions for clinical care. Tailoring medical treatments for people who are deaf, hard-of- hearing, or presenting with sensory or communication disorders requires an ability to identify specific populations based on their biology to help determine their risk of developing certain conditions and predict their response to a specific treatment. Behavioral, environmental, and lifestyle data are important determinants of health status and should also be considered in precision medicine strategies. NIDCD encourages research to promote the identification of specific subpopulations based on biomarkers, risk factors, and other assessments that would improve diagnostic accuracy and facilitate development of targeted therapies.
Imagine the Future : Doctors can identify young children who have an increased likelihood of developing minimally verbal autism to begin targeted and appropriate interventions for effective communication.
Goal 1: Accelerate the acquisition and ethical use of genetic and phenotypic data.
Comprehensive datasets such as whole-exome and whole-genome sequencing; linkage analyses; epigenetic and gene expression profiles; tissue banks; blood and serum samples; biopsy specimens; electronic health records; and lifestyle and behavioral or social-factor data are needed for scientists to advance precision medicine research efforts. These datasets should be accessible to the scientific community, but in the context of respect for and protection of an individual’s privacy and confidentiality. All researchers must consider the stewardship, intellectual property, reciprocity, and ethical use of these data. With these considerations in mind, NIDCD encourages research that will obtain, characterize, and catalog data on the impact of and interactions between genetic, phenotypic, and biological data and clinical, environmental, behavioral, social, and lifestyle factors, to serve as a resource to advance the understanding of the various forms of deafness, communication, and other sensory disorders.
Goal 2: Develop genetic and cellular therapies.
NIDCD seeks research that leads to the development of targeted treatments including delivery vehicles, gene editing tools, gene repair/correction, and site-specific, controlled, and sustained molecular therapies for communication disorders. This goal includes development of therapies to prevent loss of sensory function and/or to restore function after loss has occurred. Further, targeted molecular and cellular therapies are needed to improve neuronal function, resist cell damage from internal and external stressors, and enhance cell repair. NIDCD also encourages studies designed to elucidate the properties that enable stem cells in the sensory organs and the central and peripheral nervous systems to proliferate and differentiate, advancing not only the treatment of sensory and communication disorders, but also other neurological diseases. While NIDCD encourages targeted approaches to treatment, the Institute also encourages and values development of treatments that can impact different disorders that share common phenotypes (observable characteristics.)
Imagine the Future : People using prescription drugs to treat cancer or serious infections receive an additional treatment to protect their hearing from being damaged by these lifesaving drugs.
Goal 3: Identify and develop interventions targeted to specific subpopulations.
Precision medicine approaches for prevention, detection, and treatment of disorders and conditions have immense potential to improve health outcomes for individuals who are deaf, hard-of-hearing, or presenting with other communication disorders. Such approaches will allow clinicians to select specific treatments based on an individual’s genetic, molecular, physiological, imaging results, or other biomarkers. NIDCD encourages research to develop interventions that can be applied to subpopulations, with consideration of environmental and social differences, and that may result in improved individualized clinical care and better quality of life for those with sensory and communication disorders or conditions.
NIDCD encourages the translation of basic biomedical or behavioral research discoveries into new clinical tools, prostheses, assistive devices, behavioral therapies or interventions, and medications to ameliorate communication disorders. Translational research is facilitated by collaborations and interactions between basic scientists and clinical scientists. These interactions provide the platform for the bidirectional exchange of ideas, stimulate new avenues of research, and accelerate the advancement of basic research findings into the clinic.
NIDCD recognizes that increased dissemination and implementation research is needed to bridge the gaps between basic research, clinical implementation, and public health policy, by building a knowledge base about how health information, effective interventions, new clinical practices, and guidelines and policies are communicated and integrated into public health and health care service use in specific settings. Despite scientific and technological discoveries that have improved the health of the U.S. population overall, racial and ethnic minorities, persons with disabilities, socioeconomically disadvantaged populations, underserved rural populations, and sexual and gender minorities experience health disparities and continue to bear disproportionate burdens of disease and illness. NIDCD encourages research to improve the health, emotional well-being, and quality of life of all individuals impacted by conditions and disorders that fall within the NIDCD mission.
Imagine the Future : Vaccines that prevent otitis media (ear infections) eliminate this major cause of hearing loss, ear disease, pain, and stress for children and their families.
Goal 1: Accelerate the development of treatments.
Significant scientific advances have improved our understanding of the pathophysiology of many communication disorders. In contrast, clinicians struggle to treat patients who have disorders for which the underlying mechanisms are unknown. The scientific community is poised to develop better treatment and prevention strategies for many disorders in our mission areas using innovative approaches and leveraging knowledge and new technologies from related fields of research. NIDCD sees great opportunities to improve human health in areas such as vaccine development, collaborations between interdisciplinary scientists, novel interventions, repurposing of drugs approved by the U.S. Food and Drug Administration (FDA), bench-to-bedside research, and bedside-to-bench research to prevent and treat communication disorders. NIDCD seeks research that uses the Small Business Innovation Research/Small Business Technology Transfer Programs to commercialize novel therapies and devices to treat or prevent communication disorders. NIDCD encourages effective treatments that consider a whole person approach and additional biological, behavioral, psychological, and social research on both humans and animal models.
Goal 2: Develop, disseminate, and implement evidence-based practices to improve health-related outcomes.
NIDCD aims to support research that will close the gap between basic biomedical and behavioral discovery and population health and health care delivery for deafness and communication disorders. When developing evidence-based practices, researchers should consider racial, ethnic, and cultural diversity, as well as disability, gender, and other social determinants of health such as socioeconomic status and education. NIDCD encourages dissemination and implementation research to identify, understand, and address barriers to the adoption, adaptation, sustainability, and integration of evidence-based interventions, tools, and policies.
Imagine the Future : Medical providers routinely use smell tests to identify people more likely to develop dementia and other neurodegenerative disorders.
Goal 3: Promote health equity and improve access to clinical care.
NIDCD seeks to support more research that advances the understanding or reduces the impact of health disparities and inequities in communication disorders among racial/ethnic minorities and other underrepresented populations. Tackling the complex drivers of health disparities requires strong partnerships among researchers, community representatives, community organizations, health service providers, public health agencies, and policymakers to ensure that relevant and culturally and contextually appropriate research is conducted, and that findings can be translated into sustainable community- and system-level changes that promote health equity. NIDCD wants to ensure that individuals who are deaf, hard-of-hearing, or presenting with communication disorders are active participants in the research. Their voices, experiences, and perspectives are critically important to the development of equity, accessibility, and person-centered care. NIDCD encourages innovative clinical and translational research that provides the foundation for improving accessible and affordable health care for these individuals.
In alignment with NIH-wide priorities outlined in the NIH Strategic Plan for Data Science , NIDCD seeks to maximize the value of data generated through NIDCD-funded research by prioritizing efforts to share biomedical and behavioral data in ways that encourage ongoing use by the entire scientific community. Widespread access to shared data, along with other advancements in data science, create a unique opportunity to accelerate the discovery of insights that will improve the lives of millions of people with communication disorders. Shared scientific data accelerate biomedical research discovery, enable validation of research results by independent groups, improve accessibility to large datasets that allow researchers to address high impact questions through secondary analyses, and promote data reuse across research studies. The data in repositories— such as data from model organisms; clinical, observational, and longitudinal studies; patient registries; and population-based epidemiological and statistical studies —should be standardized and structured in such a way that they are usable and interoperable with other repositories. Standard data elements that use controlled vocabularies or ontologies enable the use of machine learning to mine existing data. NIDCD encourages the management and sharing of scientific data generated from research.
Imagine the Future : Selecting targeted therapies for aphasia is made possible by artificial intelligence (AI)-assisted diagnostic tools.
Goal 1: Inform the development and use of standardized outcome measures for basic and clinical research.
In recent years, progress in basic research has resulted in identification of underlying disease mechanisms in several of the NIDCD mission areas. While this progress is highly encouraging, one obstacle to the demonstration of efficacy in human trials has been the lack of widely accepted test strategies and endpoints to assess improvement in function. The development of standardized outcome measures in basic and clinical research will allow for unbiased analysis, interpretation, and reporting of results. Such standardized measures of behavioral and health outcomes will facilitate cross-study comparisons and improve the interpretability of research findings and translation into evidence-based clinical practice. NIDCD encourages the development of standardized outcome measures that are quantifiable, objective, and correlate with clinically meaningful changes in the underlying condition to create opportunities for comparison or combination of data across studies and lend statistical power to subgroup analysis.
Goal 2: Encourage the use of data repositories to share findable, accessible, interoperable, and reusable (FAIR) data.
Biomedical research generates data for multiple variables and produces large datasets. By adhering to FAIR data principles, researchers and data scientists establish a culture of data sharing that fully leverages these valuable resources. The FAIR principles, using data and metadata standards (ontologies, taxonomies, terminologies), facilitate reuse of data and the ability to combine data from different sources to support inquiry using modern computational tools. FAIR data allows reposited datasets to be maintained, queried, and reconfigured to facilitate new analyses, which contributes to a cost-effective and efficient resource for advancing research. NIDCD will continue to partner with the NIH Office of Data Science Strategy in activities aimed at modernizing the biomedical research data ecosystem. NIDCD encourages investigators to generate biomedical research data that adheres to FAIR principles.
Goal 3: Develop artificial intelligence and machine learning algorithms that provide novel insights and applications for prevention, diagnosis, and treatment.
Computational approaches that include artificial intelligence and machine learning (AI/ML) technologies present a tremendous opportunity for data-driven discovery across the NIDCD’s mission areas. AI/ML approaches enable researchers to recognize patterns in large volumes of data, extract relationships between complex features in the data, and identify characteristics in data (including images) that were not previously discerned by conventional analytic processes. AI/ML technologies, which includes deep learning and neural networks, are quickly becoming an integrated part of many areas of biomedical, behavioral, and clinical research. AI/ML can be applied to large quantities of biomedical data to generate new insights and applications for improved prevention, diagnosis, and treatment of communication disorders.
As new sources of biomedical and health data emerge, the amount of information will continue to grow faster than can be interrogated using conventional methods. AI/ML will be an essential tool for processing, aggregating, and analyzing the vast amounts of information to drive discovery and improve patient care. NIDCD encourages multidisciplinary collaborations among computer or information scientists; engineers; and social, behavioral, biomedical, cognitive, and/or economic scientists to improve the fundamental understanding of biomedical and health-related processes related to NIDCD’s mission.
Technology has become integral to our lives, and its use is exploding in biomedical and behavioral research and in health care practice. Emerging technology has created critical tools, including instrumentation, methods, and software that can be applied to a wide variety of challenges in sensory and communication sciences. NIDCD recognizes the importance of seeking input from potential users of the technology, including individuals who are deaf, hard-of-hearing, or presenting with communication disorders. NIDCD encourages development of new technology to prevent disease; improve diagnoses, clinical decision-making, and treatment; and facilitate communication, ultimately leading to better health outcomes and quality of life.
Imagine the Future : People who are nonverbal express themselves in real time using a personalized augmentative and alternative communication (AAC) system that is portable and lightweight.
Goal 1: Employ rational design principles to engineer novel solutions.
Advances in biomedical engineering, coupled with our ever-increasing understanding of complex biological systems, can help to develop novel approaches to restoring function. Our ever-increasing understanding of complex biological systems and biomedical engineering can be used to conceptualize novel approaches that restore function. Human factors are also complex and often play a significant role in the adoption and sustained use of new technologies. New clinical tools, prostheses, assistive devices, medications, and behavioral therapies should be developed as the technology needed to meet these requirements is identified. Rational design principles are based on systematic examination of alternatives, identification of variables that can be controlled, and establishment of appropriate boundary conditions. This process can lead to, for example, devices specialized for safe and prolonged drug delivery to the human inner ear, neural prostheses based on optical stimulation, and smart biomaterials that serve as a scaffold to encourage regeneration and repair of tissue. NIDCD encourages projects that use rational design principles to engineer therapies based on health and disease knowledge that continues to emerge from basic research.
Goal 2: Enhance augmentative and alternative communication capabilities.
Advances in technology have the potential to play an ever-increasing role in augmentative and alternative communication (AAC) devices that assist people who have complex communication needs. Innovations such as brain computer interfaces, which are based on computer translation of changes in brain activity derived from a user’s intent, are examples of the potential these technologies hold for improving communication. Similarly, NIDCD seeks to support novel approaches to AAC integration. NIDCD recognizes AI/ML as tools that can facilitate normal communication and improve accessibility for those with communication disorders. Leveraging existing AI/ML for AAC devices expands the ease with which treatment strategies may be adopted by individuals who are deaf, hard-of-hearing, or presenting with communication disorders, as well as their families and caregivers. For children who have hearing loss or speech and language disorders, AAC devices can provide tailored interventions to match the context of the child’s environment and integration into the child’s acquisition of language. NIDCD encourages multidisciplinary teams that leverage expertise in neuroscience, engineering, and social and behavioral science to help millions worldwide who cannot rely on their hearing or speech to communicate.
Goal 3: Develop specialized in vivo imaging capabilities to improve diagnosis and treatment.
NIDCD seeks to enhance the development and application of advanced imaging and computational approaches to disorders of human communication. In vivo imaging is an essential component in the development of biological advances and the clinical application of treatments of communication disorders. Systems-wide studies of the molecular and cellular pathways associated with NIDCD-related research areas will require that innovative imaging solutions be developed in a highly multidisciplinary environment. NIDCD encourages research technologies that enable imaging of living human tissue relevant to communication and communication disorders to enable precise diagnosis and the development of new and targeted treatments.
Imagine the Future : People with inner ear disease see their health care provider for live imaging to enable precision diagnosis and treatment.
NIDCD will track progress on the themes and goals of this plan. Examples of progress may be new research advances, funding opportunities, research projects, workshops, collaborative efforts, etc. NIDCD will share updates on our website.
In the fall of 2019, the NIDCD’s Science Policy and Planning Branch (SPPB) began the process of updating the 2017-2021 NIDCD Strategic Plan for research. NIDCD formed an Internal Strategic Plan Working Group (ISPWG) of NIDCD staff to guide the process. Membership of ISPWG included representation from all NIDCD divisions. ISPWG’s roster is included below. In January 2020, ISPWG presented the strategic plan process and timeline to the NDCD Advisory Council.
NIDCD published a Request for Information ( NOT-DC-20-001 ) in the NIH Guide for Grants and Contracts and in the Federal Register ( FR Doc. 2020-01480 ) in early 2020 with five broad questions designed to solicit input about unmet public health needs and the public’s ideas for how to advance NIDCD’s research mission. This Request for Information was open for 60 days. SPPB reviewed all (383) submissions and summarized them for internal consideration as the Institute developed the new plan.
NIDCD performed several analyses to help guide decision-making during the strategic planning process. SPPB conducted a portfolio analysis (start at the 19 minute 23 second mark) of NIDCD-funded research projects and tied the project to Priority Areas in the 2017-2021 NIDCD Strategic Plan to describe what NIDCD is currently funding and to track progress. In order to describe the public health impact of disorders and conditions within NIDCD’s mission areas, SPPB performed two additional analyses, including a literature review to describe disease frequency estimates for hearing, balance, taste, smell, voice, speech, and language disorders, and a collaboration with the Centers for Disease Control and Prevention’s (CDC’s) National Center for Health Statistics to estimate the annual number of physician visits based on ICD, or International Classification of Diseases codes for the most common communication and sensory disorders. ISPWG presented the results from these analyses to the NDCD Advisory Council and to other scientific experts, members of the public, advocacy groups, and professional organizations at a public session of the Council.
The COVID-19 pandemic caused significant delays in the strategic planning process by preventing NIDCD from hosting in-person meetings to obtain input. NIDCD used a virtual crowdsourcing methodology based on Open Space Technology principles to gather ideas from scientific subject matter experts in the NIDCD’s seven mission areas.
NIDCD hosted a virtual kickoff meeting in July 2021. ISPWG sought broad perspective by inviting more than 200 subject matter experts representing the extramural scientific community, program officials, and scientists from other NIH Institutes and Centers, two NDCD Advisory Council liaisons, and representatives from the FDA, CDC, Department of Veterans Affairs, Health Resources and Services Administration, Department of Defense, and the Centers for Medicare and Medicaid Services. In selecting the group, NIDCD considered career stages, educational levels, geographic locations, demographic information, gender, disability status, and under-represented minority representation.
At the July kickoff meeting, Dr. Tucci described the NIDCD’s grassroots approach to developing the strategic plan, presented the results of the analyses described in the pre-planning phase, and invited the attendees to submit visionary ideas that would advance the NIDCD mission. The subject matter experts were asked to keep the public health needs in mind when formulating ideas. Dr. Tucci also presented an idea-collection template that NIDCD shared with invitees (OMB # 0925-0766) and asked them to collaborate and distribute this template to their colleagues in other disciplines and in other fields of science. In December 2021, a subset of more than 100 subject matter experts was invited to a virtual idea-generation meeting to further discuss and refine submitted ideas and to generate new ones.
Drafting and Publishing the Strategic Plan
From January to April 2022, ISPWG reviewed and analyzed all the crowdsourced ideas and used them to develop strategic themes and goals for the plan. The draft themes and goals were made available for a 30-day public comment period on the NIDCD website in May 2022. To announce the public comment period, NIDCD published a Notice in the NIH Guide for Grants and Contracts ( NOT-DC-22-008 ) and a Notice in the Federal Register ( FR Doc. 2022-09317 ). NIDCD received 379 comments from the public. After appropriate public comment input was incorporated into the draft themes and goals, ISPWG developed a full draft 2023–2027 Strategic Plan that was reviewed and approved by NIDCD staff, NIH leadership, and finalized for publication on the NIDCD website in late fall of 2022.
Nirupa Chaudhari, Ph.D. Professor, Physiology & Biophysics University of Miami School of Medicine
Dan H. Sanes, Ph.D. Professor Center for Neural Science New York University
| Wade Chien, M.D., FACS | Inner Ear Gene Therapy Program |
| Laura Cole, Ph.D. | Science Policy and Planning Branch |
| Judith Cooper, Ph.D. | Office of the Director |
| Lisa Cunningham, Ph.D. | Office of the Scientific Director and Section on Sensory Cell Biology |
| Janet Cyr, Ph.D. | Division of Scientific Programs |
| Joanne Karimbakas, M.S., R.D.N. | Office of Health Communication and Public Liaison |
| Lisa Kennedy, Ph.D. | Science Policy and Planning Branch |
| Kelly King, Au.D., Ph.D. | Division of Scientific Programs |
| Roger Miller, Ph.D. | Division of Scientific Programs |
| Cathy Rowe, B.S. | Science Policy and Planning Branch |
| Elka Scordalakes, Ph.D. | Science Policy and Planning Branch |
| Melissa Stick, Ph.D., M.P.H. | Scientific Review Branch |
| Susan Sullivan, Ph.D. | Division of Scientific Programs |
| Debara Tucci, M.D., M.S., M.B.A. | Office of the Director |
| Becky Wagenaar-Miller, Ph.D. | Division of Extramural Activities |
| Timothy Wheeles, M.A. | Office of Administration |
| Baldwin Wong, B.S. | Science Policy and Planning Branch |
* Note: PDF files require a viewer such as the free Adobe Reader .

- Mission and Vision
- Scientific Advancement Plan
- Science Visioning
- Research Framework
- Minority Health and Health Disparities Definitions
- Organizational Structure
- Staff Directory
- About the Director
- Director’s Messages
- News Mentions
- Presentations
- Selected Publications
- Director's Laboratory
- Congressional Justification
- Congressional Testimony
- Legislative History
- NIH Minority Health and Health Disparities Strategic Plan 2021-2025
- Minority Health and Health Disparities: Definitions and Parameters
- NIH and HHS Commitment
- Foundation for Planning
- Structure of This Plan
- Strategic Plan Categories
- Summary of Categories and Goals
- Scientific Goals, Research Strategies, and Priority Areas
- Research-Sustaining Activities: Goals, Strategies, and Priority Areas
- Outreach, Collaboration, and Dissemination: Goals and Strategies
- Leap Forward Research Challenge
- Future Plans
- Research Interest Areas
- Research Centers
- Research Endowment
- Community Based Participatory Research Program (CBPR)
- SBIR/STTR: Small Business Innovation/Tech Transfer
- Solicited and Investigator-Initiated Research Project Grants
- Scientific Conferences
- Training and Career Development
- Loan Repayment Program (LRP)
- Data Management and Sharing
- Social and Behavioral Sciences
- Population and Community Health Sciences
- Epidemiology and Genetics
- Medical Research Scholars Program (MRSP)
- Coleman Research Innovation Award
- Health Disparities Interest Group
- Art Challenge
- Breathe Better Network
- Healthy Hearts Network
- DEBUT Challenge
- Healthy Mind Initiative
- Mental Health Essay Contest
- Science Day for Students at NIH
- Fuel Up to Play 60 en Español
- Brother, You're on My Mind
- Celebrating National Minority Health Month
- Reaching People in Multiple Languages
- Funding Strategy
- Active Funding Opportunities
- Expired Funding Opportunities
- Technical Assistance Webinars

- Community Health and Population Sciences
- Clinical and Health Services Research
- Integrative Biological and Behavioral Sciences
- Intramural Research Program
- Training and Diverse Workforce Development
- Inside NIMHD
- ScHARe HDPulse PhenX SDOH Toolkit Understanding Health Disparities For Research Applicants For Research Grantees Research and Training Programs Reports and Data Resources Health Information for the Public Science Education

- Understanding Health Disparities Series
- Diversity & Inclusion in Clinical Trials

- News Releases
- NIMHD in the News
- Conferences & Events
- Research Spotlights
- E-Newsletter
- Grantee Recognition
Diversity and Inclusion in Clinical Trials
Go to diversity and inclusion in clinical trials scientific resources
Our health is a combination of physical and mental well-being, which is affected by our behavior, biology, environment, societal policies, and importantly, our lived experiences. The lived experiences of people in the United States vary based on their race and ethnicity, socioeconomic status (SES), geographic location, sexual orientation, gender identity, and other sociodemographic characteristics.
Lived experiences also need to be understood in the context of the individual and structural social determinants of health.
How and where we live, learn, work and play, and our access to high quality health care, healthy foods, and quality education can enhance our health outcomes.
Similarly, negative experiences and exposures, such as pollution, violence, and structural racism and discrimination, can negatively affect our health.
Our health status reflects the interwoven effects of such factors.
A clinical trial is a type of clinical research that evaluates the effects of intervention(s), including drugs, devices, surgeries, diets, behavioral approaches, and lifestyle interventions, on health-related biomedical or behavioral outcomes.
To account for the diverse lived experiences and exposures of various populations, clinical research should be appropriately inclusive of racial and ethnic minority groups, as well as other populations experiencing health disparities, including sexual and gender minority or socioeconomically disadvantaged populations.
Clinical trials evaluate the safety and effectiveness of medical treatments and devices, including drugs, surgeries, diets, behavioral approaches, and interventions in lifestyle, to improve individual and community health.
To account for the diverse lived experiences and exposures of the general population, clinical trials must be appropriately inclusive of racially and ethnically diverse population groups, as well as other vulnerable groups including sexual and gender minority or socioeconomically disadvantaged populations, to understand the effects of SDOH on health outcomes.
Why Are Clinical Trials Important?
Clinical trials can:
- Determine if a new intervention is safe, works better, and/or has fewer side effects than an existing treatment or intervention.
- Examine ways to detect a disease early, when it is potentially more treatable, or ways to prevent a health problem altogether.
- Evaluate ways to improve the quality of life of people who have an illness or chronic medical condition.
- Include testing of behavioral, social, environmental, and structural interventions.
Participating in clinical trials is voluntary. People volunteer to participate in clinical trials for a variety of reasons.
- One of the most common reasons is altruism—the opportunity to contribute to science and the common good and/or help those with similar health issues.
- People may volunteer when it allows them to receive an experimental intervention for life-threatening or disabling disease where no standard treatments are available or were already tried without success.
- New interventions (e.g., weight loss or tobacco cessation interventions) that haven’t yet been approved by the U.S. Food and Drug Administration (FDA) may be tested for common conditions to understand if the intervention might help a condition in situations where current treatments or interventions don’t exist, don’t work well, or have unwanted side effects, or provide symptomatic relief, but offer no cure.
The Importance of Diversity & Inclusion in Clinical Trials
People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities can benefit from scientific advances.
Factors that can influence the risk and likelihood of developing a disease, experiencing a long-term health outcome, and responding to treatment include (but are not limited to):
- Biological sex
- Pregnancy status
- Life experiences (negatives, such as psychosocial stress and lack of basic resources, or positives, such as educational and employment opportunities)
- Unhealthy behaviors (e.g., substance use, sedentary lifestyle, overeating, risky sexual activity)
- Health-promoting behaviors (e.g., adequate sleep, obtaining recommended preventive services, physical activity, healthy eating)
- Environmental conditions (e.g., pollution, access to health care or healthy foods, neighborhood segregation)
- Genetic variation and geographic ancestry
- Underlying medical problems or presence of comorbidities (i.e., additional diseases or conditions)
Historically, clinical trials did not always recruit participants who represented the individuals most affected by a particular disease, condition, or behavior. Often, these clinical trials relied almost exclusively on White male study participants. This shortcoming has created gaps in our understanding of diseases and conditions, preventive factors, and treatment effectiveness across populations. These gaps in knowledge can impede the quality of health care decision making, ability to counsel people on ways to reduce their risk, optimal treatment responses, and even the development of more effective medications or interventions.
Clinicians and researchers should carefully consider the inclusion or exclusion criteria for their clinical trials. For example, a clinical trial excluding participants with high blood pressure or other comorbidities may end up excluding many people over 65 years old, who are more likely to have these conditions. The trial may then underrepresent certain groups in the study and make the results less applicable to groups who may benefit the most from the findings.
Coronavirus disease 19 (COVID-19) has disproportionally affected racially and ethnically diverse populations, including African American, Hispanic/Latino, and American Indian/Alaska Native individuals, who are three times more likely to be hospitalized than White individuals.
Real-World Examples of the Need for Inclusion in Clinical Trials
Understanding covid-19 disparities.
During the early stages of the pandemic, Coronavirus disease 2019 (COVID-19) disproportionately affected racial and ethnic minority populations, including African American, Hispanic/Latino, American Indian/Alaska Native, and Native Hawaiian and Pacific Islander population groups, with increased cases, hospitalizations and deaths.
It was critical that COVID-19 vaccine trials included sufficient representation across population groups to better understand vaccine effectiveness in populations who vary on environmental exposures and other lived experiences. By using inclusive recruitment practices in COVID-19 clinical trials, researchers have been able to show that vaccine safety and efficacy are similar across all racial and ethnic populations. Engaging diverse populations in planning and implementing such trials can also help increase public confidence that the vaccine is safe and effective.
Understanding Asthma Disparities
Asthma disparities are intricately linked with the environment. Living in a city may increase exposure to air pollution and risk for developing asthma. Exposure to tobacco smoke, chronic social stress , or unhealthy diets may also influence asthma risk or severity. Thus, it is vital for clinicians and researchers to consider where patients live, what they eat, and how they feel—as well as characteristics like race, ethnicity, socioeconomic status, and age—to get a more thorough understanding of their patients’ experience with asthma symptoms and identify the best preventative strategies or treatment options.
Asthma disparities are intricately linked with the environment. Living in a city may increase exposure to air pollution and risk for developing asthma. Exposure to chronic social stress or unhealthy diets may also influence asthma risk or severity. Thus, it is vital for doctors to include study conditions like where patients live, what they eat, and how they feel—as well as fixed characteristics like race, ethnicity, and age—to get a more thorough understanding of their patients’ illness. This knowledge can help doctors find and test which preventative strategies or treatments work best for different patients with asthma.
Why Diversity in Clinical Trials Is a Social Determinant of Health
Inclusive participation in clinical trials benefits scientific discovery.
NIH is committed to inclusivity in clinical trial research . It is essential to have a wide range of people from different communities participate in clinical trials to reduce biases, promote social justice and health equity, and produce more innovative science. Below is a list of topics and examples to illustrate the important role of inclusive participation in clinical trial research.
Countering Mistrust in Clinical Research
Historical atrocities and incidents have engendered mistrust in clinical research and medical institutions.
Investigators conducting the U.S. Public Health Service Syphilis Study at Tuskegee between 1932 and 1972 did not explain the study’s risks and obtain formal agreements (called informed consent ) from the African American men who were its participants. The researchers wanted to study the effects of untreated syphilis and withheld penicillin treatment when it became available in 1945, which would have helped the 399 study participants with the disease. Only when news leaked of the study in 1972 did their unethical and discriminatory behavior come to light. Their actions caused preventable illness and death in study participants and their families.
In 2003, members of the Havasupai Tribe in Northern Arizona learned that DNA samples given in the early 1990’s for a diabetes research study were later being used for additional research on ethnic migration, schizophrenia, and other unrelated genetic studies. The informed consent form from the original study did not ask participants for their permission to use these samples for these other analyses. The researchers failed to obtain their consent for use of their data and specimens for other research purposes.
The failings of the Syphilis Study at Tuskegee contributed to the creation of the Belmont Report in 1976, which addresses ethical issues in research with human participants. It outlines basic ethical principles and essential guidelines to protect human research participants and ensure safety in clinical trial research.
Today, Institutional Review Boards are responsible for reviewing all studies involving humans for compliance with these guidelines and reports of any study protocol violations. In recent years, people from racial and ethnic minority communities and other populations experiencing health disparities have become more willing to participate in clinical research . Developing trust with communities who have been marginalized is best achieved through meaningful partnerships between researchers and community members in planning and carrying out studies with their input.
Inclusion of Women and People from Racial and Ethnic Minority Groups in Clinical Trials
The NIH Revitalization of Act of 1993 was signed into law, authorizing NIH to continue its mission and importantly establishing guidelines for the inclusion of women and persons from racial and ethnic minority populations in clinical research. The goal of this law, and other guidelines, is for clinical trial participants to adequately reflect the diversity of the real-world population, so that researchers can determine whether the variables being studied affect women or members of any racial and ethnic population group. This helps ensure that research findings are generalizable to the entire population.
NIH efforts toward research inclusion remain at the forefront of clinical research policy . Recent activities include the publicly available NIH Research, Conditions and Disease Categorization Inclusion Statistics Report , which provides data on human research participation in NIH clinical research studies by race, ethnicity, and sex/gender. Additionally, in 2017, NIH updated its policy on the inclusion of women and people from racial and ethnic minority populations with a requirement that "recipients conducting applicable NIH-defined Phase III clinical trials ensure results of valid analyses by sex/gender, race, and/or ethnicity are submitted to Clinicaltrials.gov ." See NIH Inclusion Outreach Toolkit: How to Engage, Recruit, and Retain Women in Clinical Research for more information.
Inclusion of Sexual and Gender Minority Populations
Until recently, health care systems and epidemiological surveys often didn’t ask sexual orientation and gender identity questions to consider inclusion of sexual and gender minority (SGM) persons. This has made it difficult to know if individuals within SGM populations are represented in clinical research studies in significant numbers to make results representative for them. This lack of knowledge can influence patient-clinician communication and can result in fewer health screening or treatment opportunities .
This lack of knowledge can influence patient-clinician communication and can result in fewer health screening or treatment opportunities . This has contributed to disproportionally higher rates of anxiety, depression, and selected cancers in SGM populations and exemplifies the need to include their experiences in clinical trials and research.
Inclusion by Socioeconomic Status (SES)
An individual’s SES is a major predictor of health outcomes, because it can impact access to health care, nutritious foods, prescription medications, and other resources for healthy living. Yet, SES measures (i.e., education and income level) are not collected routinely and reported in clinical trials.
In an analysis of all randomized clinical trials published in 2015 and 2019 in the Journal of the American Medical Association, The Lancet , and the New England Journal of Medicine , study investigators reported that less than 15% of studies reported on the SES of trial participants. Lack of data collection and reporting on SES measures make it difficult to generalize research findings to all SES groups or to tailor interventions (e.g., new medications or other treatment interventions) to people with lower SES who may not be able to access or maximize the benefits of clinical trial outcomes. In addition, limited access to socioeconomic resources may pose a barrier to participation in clinical trials.
To ensure the inclusion and representation of participants across different SES levels in clinical trials, researchers should use appropriate data collection and reporting protocols. For example, NIMHD supported a social determinants of health collection in the PhenX Toolkit that includes established instruments for conducting research with human participants, such as clinical trials.
Researchers should also design their studies and provide resources to make it easier for people with lower SES to participate in clinical trials, such as offering convenient locations and hours of operation, childcare services, and transportation vouchers.
Data Collection and Reporting: Unmasking Hidden Truths
When scientists combine information from individual research participants, this is called data aggregation. Data aggregation is an important part of the research process that protects the anonymity of research volunteers and strengthens the statistical analysis of the study. However, aggregation of demographic data, including race and ethnicity, can also mask important differences in health risks or outcomes for specific subpopulations.
For example, many prior studies on the health of Asian Americans have not always examined differences by nationality. A recent study found that among Filipino, Vietnamese, Chinese, Japanese, and Korean American adults living in California, categorizing all participants as ”Asian American” masked at least one health disparity for each subpopulation.
Clinicians and researchers must take care to define as best as possible the clinical trial sample in their studies and consider whether their findings can be generalized across population groups, including consideration for differences in lived experiences.
NIMHD is studying and addressing issues related to diversity and inclusion in clinical trials through a variety of strategic goals, funding initiatives, and educational materials:
NIMHD Content & Resources
Nih minority health & health disparities strategic plan 2021-2025.
The National Institutes of Health Minority Health and Health Disparities Strategic Plan 2021-2025 was developed by NIMHD, in collaboration with all the NIH Institutes, to outline NIH’s commitment to improving minority health and reducing health disparities. Two of the Strategic Plan’s major goals focus on issues of diversity and inclusion in NIH-funded research and in clinical trials.
- Goal 7: Ensure appropriate representation of minority and other population experiencing health disparities in NIH-funded research
- Goal 8: Promote evidence-based community engagement, dissemination, and implementation of minority health and health disparities research best practices
The Strategic Plan’s Leap Forward Research Challenges are goals that aim to redefine the science of minority health and health disparities. These goals include:
- Increase the overall proportion of participants from diverse populations included in NIH-funded clinical research to 40 percent by 2030 and within specific major disease categories.
- Increase the diversity of institutions conducting genomic research and training by investing in faculty at such institutions, along with curriculum-building partnerships, to accelerate workforce development in underrepresented and under-resourced communities within the next 10 years.
NIMHD Articles
Research features.
- Q&A: Tuskegee University Researchers Embrace History and Community
- Patient Navigators: The Missing Link to Increasing Minority Participation in Cancer Clinical Trials
- The Center for Asian Health Engages Communities in Research to Reduce Asian American Health Disparities
NIMHD Insights Blog
- The Sweetness of Our Ancestors: Thoughts on Diabetes, Genetics, and Ethnic Diversity in Research by Larissa Avilés-Santa, M.D., M.P.H.
- The Power of Trust and Truth by NHLBI Director Gary H. Gibbons, M.D. and NIMHD Director Eliseo J. Pérez-Stable, M.D.
- Communicating the Value of Race and Ethnicity in Research by NIMHD Director Eliseo J. Pérez-Stable, M.D.
- Reducing Health Disparities and Enhancing Diversity in Aging Research by National Institute on Aging Director Richard J. Hodes, M.D.
NIMHD Funding Resources & Opportunities
Funding opportunity announcements.
Clinical trials and other forms of clinical research focused on populations experiencing health disparities are vital to improving our understanding of the causes of many diseases that affect these populations. New insights from such studies can transform medical practice and health promotion in targeted populations. See Active NIMHD Funding Opportunities for more information.
NIMHD-Supported Research Projects
NIMHD sponsored a Funding Opportunity Announcement ( RFA-MD-11-005 ) to support projects that identified evidence-based strategies to increase enrollment of racial and ethnic minority persons into clinical trials. Below are examples of some of these projects with links to published findings or guidelines for best practices. Projects included:
- National Initiative for Minority Involvement in Neurological Clinical Trials (NIMICT) ( U24MD006961 ) The goal of this project was to identify and evaluate strategies to promote recruitment and retention of racial and ethnic minority persons in neurological clinical trials. The NIMICT project generated toolkits, manuals, educational videos, and online training modules to guide investigators through the process of designing inclusive clinical trials. Project investigators:
- Worked with StrokeNet , NIH’s network of clinical trials addressing stroke, to ensure that all funded trials have acceptable racial and ethnic minority participant recruitment plans.
- Identified barriers and strategies for recruitment of racial and ethnic minority persons in neurological research.
- Examined barriers and practices for recruitment and retention of participants in stroke clinical trials.
- Enhancing Minority Participation in Clinical Trials (EMPaCT): Phase II ( U24MD006970 ) The goal of this project was to test strategies to increase racial and ethnic minority patient enrollment in cancer clinical trials. The work for this project was conducted across five National Cancer Institute Comprehensive Cancer Centers. The investigators:
- Generated strategies that facilitate patient engagement in cancer care, including understanding institutional barriers to recruitment and utilizing patient navigators to facilitate the enrollment of racial and ethnic minority individuals into cancer clinical trials.
- Examined bias and stereotyping and the impact on recruitment to cancer clinical trials.
- Identified needed training to increase cultural awareness of clinical and research professionals to help recruitment and retention in cancer trials
- Assessed the motivations of clinical and research staff for including racial and ethnic minority populations in cancer clinical trials.
NIMHD-Supported, NIH-Wide Initiatives
- The NIH RePORT on Inclusion of Women and Minorities in Clinical Research is a database that contains inclusion information on NIH-funded clinical research since 1994.
- NIH Clinical Research Trials and You features educational resources and information about diversity in research, including policies and guidance on the inclusion of women and racial and ethnic minority populations and population groups across the lifespan .
- Diversity is a core value of the NIH All of Us Research Program , which stresses the importance of the participation of people of different races, ethnicities, age groups, regions across the country, gender identities, sexual orientations, socioeconomic backgrounds, disabilities, and health status.
- NIH Inclusion Data by Research and Disease Category Now Available – NIH Extramural Nexus
- The National Institute on Aging’s Recruiting Older Adults in Research (ROAR) Toolkit .
- The National Institute of Mental Health’s Points to Consider about Recruitment and Retention While Preparing a Clinical Research Study .
- The National Center for Advancing Translational Sciences Trial Innovation Network .
- The Community Engagement Alliance (CEAL) Against COVID-19 Disparities provides resources to promote and ensure diversity and inclusion in vaccine and diagnostic testing .
Additional Resources
- The Food and Drug Administration’s Clinical Trial Diversity webpages include resources such as:
- Clinical Trial Diversity Fact Sheet
- Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry
- Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups | The National Academies Press
Page updated Jan. 12, 2024
NIH Inclusion Policies
FDA Statement on Diversity in Clinical Trials
NIH UNITE Initiative
NIH CEAL: Diversity & Inclusion
Understanding Health Disparities: Structural Racism and Discrimination
NIMHD Fact Sheet
Read about what is happening at NIMHD at the News and Events section
301-402-1366
Connect with Us
Subscribe to email updates

Staying Connected
- Funding Opportunities
- News & Events
- HHS Vulnerability Disclosure
- Privacy/Disclaimer/Accessibility Policy
- Viewers & Players
- Search Menu
- Sign in through your institution
- Author videos
- ESC Content Collections
- Supplements
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- About European Heart Journal Supplements
- About the European Society of Cardiology
- ESC Publications
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, defining the plan: mission, vision, and goals, organizational culture, clinical program, performance, and reporting to drive improvement, clinical research, geographic network, building an enabling information technology.
- < Previous
Strategic management of a healthcare organization: engagement, behavioural indicators, and clinical performance
- Article contents
- Figures & tables
- Supplementary Data
Giuseppe Speziale, Strategic management of a healthcare organization: engagement, behavioural indicators, and clinical performance, European Heart Journal Supplements , Volume 17, Issue suppl_A, March 2015, Pages A3–A7, https://doi.org/10.1093/eurheartj/suv003
- Permissions Icon Permissions
Healthcare organizations today are facing a series of problems due to two main factors: increasing difficulty in satisfying a progressively more ‘aware’ and demanding user, and the need to change their internal organization to keep pace with the very rapid changes taking place in technology and approach. A continuous increase of complexity and the capacity of physicians will not ensure the fundamental requirement of any business: to really deliver what its customers need. Hence, it is time for a revolutionary strategy focused on: (i) maximizing value for patients by obtaining the best outcomes at lowest cost and (ii) moving from a physician-centred organization to an ‘organization-driven’ care process.
However, complex systems are typically conservative and rather resistant to change, and the healthcare system is no exception to this rule. The challenge is that doctors have to be central players in the healthcare revolution and any strategy that they do not embrace will fail. Certainly, a piecemeal approach will not work. Engaging doctors in transforming the system requires focusing on shared goals, by using motivational tools: shared purpose, peer pressure, measuring performance, and enhancing a patient-centred approach.
GVM Care & Research is a holding operating in the health, pharmaceutical, spa-well-being, research, biomedical industry, company-aimed services, real estate, and financial areas. The core business is the network of highly multi-specialized hospitals and day-hospital outpatient clinics: this complex system, involving specialized facilities and highly qualified professional expertise, is present in numerous Italian regions and extends also to France, Albania, and Poland. GVM is one of the key players in Italy in cardiac surgery (responsible for ∼15% of all cardiac interventions in Italy) and interventional cardiology, with documented excellent outcomes (cf. the Italian National Healthcare Agency and Italian Ministry of Health).
The first step in any strategic transformation is to clarify the institutional mission, visions, and goals. The ‘mission’ declares the organization's distinctive purpose or reason for being. The vision represents what its leaders want the organization to achieve when it is accomplishing the mission. Strategic goals are those overarching end results that the organization pursues to accomplish its mission. 1–4 GVM's cardiovascular mission in the past was: ‘Improve the health and well-being of patients through effective approaches to the diagnosis and treatment of cardiovascular diseases and their prevention mediated by innovative clinical research’. The strategy for moving now to a high-value healthcare organization comprises five variables: (i) designing and implementing a corporate organization dedicated to cardiovascular patients, including new clinical governance rules; (ii) driving the changes by work volume and performance, in a single matrix; (iii) increasing innovation in clinical processes and implementing clinical research as a structural component of clinical procedures; (iv) expanding geographic networking; and (v) developing an advanced information technology (IT) platform 5 ( Figure 1 ).

The GVM value agenda.
The task of building a vision for an organization is frequently referred to as ‘path-finding’. The goal of the pathfinder is to provide a vision, find the paths that the organization should propose in the long run and mark the trail for those who will follow. 6–10 To effectively outline the future and facilitate the pursuit of organizational excellence, visions need to be translated into ‘action plans', considering:
primary targets, i.e. sectors to be sustained, expanded, or reduced;
external context analysis, e.g. the presence and type of competitors, geographic and demographic data, the network and relations, and international connections;
internal context analysis, i.e. expertise, mindset and attitude of the heart team, structure, organization, quantity and quality of production (database), and periodic monitoring of the database and processes;
strategic targets, e.g. teamwork, performance improvement, increased number of patients referred, innovative techniques, inpatient clinics plan (GVM point program), innovation and production in clinical research, and presence and competitiveness in Europe.
To successfully implement the strategy, a change in ‘organizational culture’ is required. Although cultural change is difficult, it is often an important factor in moving the healthcare system toward realizing its strategy. An organizational culture is the consciousness of the organization that guides the behaviour of individuals; it may be founded on shared purpose, value, and behavioural norms. The GVM organization was created by a bottom-up approach with shared assumptions including a common understanding of ‘who we are’ and ‘what we are trying to accomplish’. Certainly, we shared values such as a common understanding of ‘GVM doing things'. Parallel to its cultural change now, GVM has adopted a new organizational structure in order to facilitate the implementation of the overall strategy 11–20 ( Figure 2 ).

Organizational structure of GVM.
The healthcare future will be based on larger and integrated systems, patient-centred care, a new relationship between hospitals and physicians, and a shift of many inpatient procedures to outpatient or home settings. 21–32 Since the biggest driver of rising costs is medical progress and procedural improvements that generate a fragmented and disorganized system, in order to create a common language, GVM has defined guidelines for a strategic plan focusing on high-quality, medically excellent procedures, innovative techniques, participation in international networks, and scientific publications.
Organizational culture requires rigorous measurements of value: namely, outcomes and costs. Accordingly, we introduced the GVM performance index, composed of the key indicators of clinical activity, approach and results pertaining to the single hospital and its surrounding area (GVM Area, see geographic network). Measuring a full set of outcomes that matter is indispensable to better meeting patients' needs. At each single level, variables (hence indexes) were grouped into four macro-areas (Table 3): (i) clinical indexes, (ii) program index, (iii) economic indexes, and (iv) reputation index ( Figure 3 ).

GVM area and hospital score indexes.
Area performances, as well as the medical team performances, should be computed on the basis of the ability of both areas and teams to reach the established targets. This approach has made possible a greater integration of production and performance data, finally available within a single matrix and, hence, more sensitive and able to describe the Group's positioning and capability, both horizontally (in a given time, between different hospitals), and vertically (in a given hospital, across different moments). Monitoring has been applied, both at single-hospital level, at hub-and-spoke area level, and at medical team level (entire network within a given medical area).
For some of the variables comprised in the above-mentioned indexes, a given numeric threshold was identified and set (in coherence with recent regulations, i.e. the ‘Balduzzi’ law), while in other cases the threshold was set at a value equal or better than GVM's average performance. Financial incentives were used in GVM in the past but they were not sufficient to optimize doctors' performance. Since comparing outcomes is complicated, we have implemented coordination, information sharing and team work as performance measures. 33–43
These systematic measurements of results and the periodic activity of reporting outcomes using peer pressure have produced significant improvement in quality of care, outcomes and costs in GVM and have positively influenced several important indicators in the cardiovascular area: As far as the area indexes are concerned, there was a clear improvement in all areas considered. Results from the Cardiac Surgery and Cardiology Hospital Score indices are also very interesting: measuring the Cardiac Surgery index, six hospitals of nine improved their performance, one showed no difference, while only two showed a lower score, due to external reimbursement regulations.
the volume of cardiovascular surgical procedures performed by GVM in 2013 rose by ∼10%, inverting the negative trend of the previous 3 years;
the average length of hospital stay in cardiac surgery decreased in 2013 by about half a day compared with the same period in the previous year;
cardiac surgery mortality decreased significantly, by 1%, in the same period;
endovascular cardiology increased by ∼1% in 2013 with respect to 2012.
Scientific research is a necessary component of a healthcare institution working in areas where culture, technology, and clinical care processes move quickly and need continuous updating, first so as to keep abreast of intellectual advances, secondly so as to be part of the community of experts able to discern the quality of the new proposals and disentangle true novelties from cosmetic changes, thirdly so as to be able to contribute to the advances and play a role in their management. Clinical research should not be a corollary in the strategic planning of health management, but rather it should be a primary component in the array of mid- to long-term goals, and also part of the investment plan.
The structure and planning of the scientific activity of the GVM network is coordinated by a Scientific Direction including: (i) a Clinical Research Unit, managing methodologically and operationally the studies, including the formal requirements (by local institutional review boards), the interaction with the investigators, and monitoring of the quality of the data; (ii) an Informatics Unit: managing data collection and database maintenance.
Platforms for clinical research include first multiple databases implemented in each main field of interest to systematically record the routine activity, with branches related to specific research protocols. Secondly, the available technology is used for implementing validated core labs for central reading of intra-vessel imaging and cardiovascular function records in blinded fashion for multi-centre trials. Finally, structured web networking allows fluid internal and external connections for meetings, webinars, scientific journals access, etc.
The current fields of interest in the GVM group are the following:
intraluminal imaging and interventional procedure technology
pathophysiology of the vascular wall (proteomics, biomarkers)
transcatheter structural heart disease repair
advanced cardiovascular surgery
cardiac electrophysiology (applied in both ablation and repair procedures)
regenerative medicine
GVM is a multisite healthcare delivery organization controlling a wide and continuously growing network of hospitals spread throughout Italy and abroad. However, the level of integration and connections between the nodes of a dynamically changing network requires periodic systematic adjustments. To improve values, eliminate fragmentation and duplication of care, and optimize organization, we have introduced the ‘hub-and-spokes' model. In this model, we define the role of each hospital, concentrating work volumes within a few hospitals, choosing the best location for each clinical approach and integrating patient care across hospitals.
The hospitals have been assigned to four different geographic areas, in relation to their location. In addition, a hub for each area has been identified, intended to act as a natural ‘centre-of-gravity’ for the network of hospitals situated within the relative geographic area. The spokes, i.e. the network of hospitals comprised within the hub's gravitational system, are directly linked to their main hub, and indirectly connected, through ‘hub-to-hub’ connections, to spokes in other areas. As far as the area working plans are concerned, it should be noted that the Italian healthcare system is strongly ‘regionalized’, both in terms of clinical and administrative organization.
Finally, several actions have been conceived and implemented to improve GVM's visibility within Italy and abroad, through a marketing and communication campaign, as well as new and innovative networking instruments, such as the GVM Point initiative. The GVM Point initiative was designed to establish a network of inpatient clinics. These clinics typically provide first-level diagnostic services. The underlying franchising-like proposal was to integrate these clinical investigations with 2nd-level, 2nd-opinion ‘heavy-machine’-based options (such us MRI-scan, CT-scan, X-ray, etc.) at GVM clinics, as well as pre-surgical consultations and planning. Local affiliates can benefit from the expertise and reputation of GVM hospitals for referral of complex cases, so improving their own status. This enables even relatively small inpatient clinics to provide patients with an almost complete range of medical services and solutions.
The core of the GVM value agenda is to support a solid IT platform. A multidisciplinary and multidimensional organization like GVM needs to be complemented by an efficient delivery system. The IT program is focused on a platform that follows patients across services, using a common data definition and containing all patient data. Healthcare IT is acknowledged as instrumental in reducing medical errors, enhancing staff productivity, improving quality, and lowering costs. The medical path is accessible to all stakeholders and by any GVM structures, facilitating patients' referral, diagnosis and treatment, and outcome and costs measurement. The global data of GVM network will be used to implement the continuous process of quality assessment and improvement, risk management and to establish a better communication with patients. 5
Hospitals and healthcare organizations are today operating in an extremely competitive environment, with increasing pressure to improve quality and reduce costs. In responding to this dynamic situation, transformation of organization requires the will to organize delivery around the needs of patients.
We have described the GVM organizational experience in reengineering the process by which care is delivered in order to make it more patient-focused. The GVM value agenda has been formulated based on mutually reinforcing components. The corporate organization has been redefined including a proper measurement of performance (outcomes and costs). An IT platform has been implemented, enhancing patient-centred vision, facilitating access to medical records for all parties involved in care, quality of care and costs. Despite the fact that the GVM is a complex and multisite healthcare organization, the strategic transformation has been carried out engaging all physicians in the total hospital network. The results at 18 months are very surprising: assessment of outcomes and costs in the cardiovascular field has shown an improvement in all GVM hospitals. 44–47
Conflict of interest: none declared.
Google Scholar
Google Preview
- organizational culture
- clinical research
| Month: | Total Views: |
|---|---|
| December 2016 | 4 |
| January 2017 | 21 |
| February 2017 | 80 |
| March 2017 | 56 |
| April 2017 | 17 |
| May 2017 | 37 |
| June 2017 | 54 |
| July 2017 | 85 |
| August 2017 | 99 |
| September 2017 | 190 |
| October 2017 | 199 |
| November 2017 | 138 |
| December 2017 | 797 |
| January 2018 | 1,182 |
| February 2018 | 1,730 |
| March 2018 | 2,632 |
| April 2018 | 2,620 |
| May 2018 | 2,489 |
| June 2018 | 2,434 |
| July 2018 | 2,240 |
| August 2018 | 2,428 |
| September 2018 | 2,467 |
| October 2018 | 2,386 |
| November 2018 | 2,834 |
| December 2018 | 2,190 |
| January 2019 | 2,515 |
| February 2019 | 3,152 |
| March 2019 | 3,591 |
| April 2019 | 3,615 |
| May 2019 | 3,796 |
| June 2019 | 3,461 |
| July 2019 | 3,744 |
| August 2019 | 3,271 |
| September 2019 | 3,325 |
| October 2019 | 2,543 |
| November 2019 | 2,659 |
| December 2019 | 2,050 |
| January 2020 | 2,261 |
| February 2020 | 2,785 |
| March 2020 | 2,687 |
| April 2020 | 3,294 |
| May 2020 | 1,711 |
| June 2020 | 2,772 |
| July 2020 | 1,825 |
| August 2020 | 1,749 |
| September 2020 | 2,172 |
| October 2020 | 1,880 |
| November 2020 | 1,805 |
| December 2020 | 1,320 |
| January 2021 | 1,406 |
| February 2021 | 1,277 |
| March 2021 | 1,841 |
| April 2021 | 1,499 |
| May 2021 | 975 |
| June 2021 | 911 |
| July 2021 | 857 |
| August 2021 | 877 |
| September 2021 | 1,170 |
| October 2021 | 1,099 |
| November 2021 | 1,036 |
| December 2021 | 823 |
| January 2022 | 831 |
| February 2022 | 915 |
| March 2022 | 1,017 |
| April 2022 | 849 |
| May 2022 | 877 |
| June 2022 | 566 |
| July 2022 | 708 |
| August 2022 | 664 |
| September 2022 | 854 |
| October 2022 | 870 |
| November 2022 | 870 |
| December 2022 | 570 |
| January 2023 | 752 |
| February 2023 | 1,104 |
| March 2023 | 999 |
| April 2023 | 702 |
| May 2023 | 483 |
| June 2023 | 387 |
| July 2023 | 505 |
| August 2023 | 559 |
| September 2023 | 711 |
| October 2023 | 641 |
| November 2023 | 650 |
| December 2023 | 557 |
| January 2024 | 527 |
| February 2024 | 509 |
| March 2024 | 935 |
| April 2024 | 585 |
| May 2024 | 507 |
| June 2024 | 367 |
| July 2024 | 430 |
| August 2024 | 130 |
Email alerts
More on this topic, related articles in pubmed, citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1554-2815
- Print ISSN 1520-765X
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- Notices of Funding Opportunities
- NIH RePORTER
- Co-Funding Activities
- BSSR Clinical Trials
- Reports and Publications
- OBSSR-Supported Training
- Online Training Resources
- OBSSR Connector Monthly Newsletter
- The Director’s Voice Blog
- Research Spotlights
- BSSR News and Announcements
- All Upcoming Events and Meetings
- NIH Behavioral and Social Sciences Research Festival
- NIH Matilda White Riley Behavioral and Social Sciences Honors
- OBSSR Director’s Webinar Series
- Mission and History
- BSSR Definition
- BSSR Accomplishments
- Strategic Plan
- NIH Behavioral and Social Sciences Research Coordinating Committee (BSSR-CC)
- Coordination of NIH-wide Initiatives
- Staff Directory

- About OBSSR
- Strategic Plan 2025-2029
Research Priorities
Strategic plan 2025–2029.
OBSSR’s fourth Strategic Plan reflects the rapidly changing nature of behavioral and social sciences research and builds on our previous accomplishments. This plan capitalizes on OBSSR’s unique coordinating role, highlighting scientific priorities that transcend specific diseases and conditions, address critical areas, and fulfill the needs of multiple NIH Institutes, Centers, and Offices (ICOs).
Select the icons to learn more about OBSSR’s strategic priorities and the cross-cutting theme for 2025–2029.

- Synergistic Inquiry
- Innovation Investigation
- Implementation and Impact: Accelerate Sustained Adoption of BSSR Finding Into Practice and Policy
- Develop and Diversify the BSSR Workforce
- Uphold Values of Diversity, Equity, Inclusion, and Accessibility (DEIA)
- Build Equitable Partnerships and Collaborations
- Enhance Communication Among Scientists and With the Public
Can we add some intro text here? Strat Plan currently does not have intro text for the Research Priorities
Research Priority 1: Synergistic Inquiry
Need intro text here – Strat Plan currently does not have an intro for this Research Priority (but does for the other two)
Goals under the Synergistic Inquiry Research Priority are to:
- Integrate and Coordinate BSSR across NIH
- Build a Cumulative BSSR Knowledge Base
- Enhance and Connect Basic and Applied BSSR
Research Priority 2: Innovative Investigation
Encouraging innovation and rigor in research measurement, experimental designs, and data analytics used in BSSR will accelerate scientific advances and support a more comprehensive and seamless integration of BSSR into the larger biomedical enterprise. Across the BSSR disciplines, there is a need to support the development and use of more advanced, multi-level, and harmonizable measurement approaches.
Goals under the Innovative Investigation Research Priority are to:
- Improve BSSR Measurement
- Promote Novel Experimental Designs
- Advance Data Analytics
Research Priority 3: Implementation and Impact
Too often there is a gap between what is efficacious in well-controlled intervention research and what works when those interventions are implemented in real-world clinical or community settings. Our previous strategic plan identified a need for more research designed to answer questions that can facilitate rapid, data-driven decisions around implementation of research and its relevance for policy recommendations and we remain committed to addressing this challenge in our current plan.
Goals under the Implementation and Impact Research Priority are to:
- Support Dissemination and Implementation Research
- Encourage the Scalability of Social and Behavioral Interventions
- Pursue Collaborations and Partnerships to Enhance Dissemination Efforts
Scientific Capacity Priorities
Can we add some intro text here? Strat Plan currently does not have intro text for the Scientific Capacity Priorities
Capacity Priority 1: Workforce Development
OBSSR recognizes the importance of developing the scientific talent and skills needed to advance health-related BSSR. Training and capacity building are foundational to an effective BSSR workforce poised to address the complex research challenges of today and the future.
Capacity Priority 2: Work Environment
We also strive to develop and diversify the workforce within our own office, as the effectiveness of OBSSR staff is crucial to the coordination and integration of BSSR across NIH and beyond.
Operational Priorities
Can we add some intro text here? Strat Plan currently does not have intro text for the Operational Priorities
Operational Priority 1: Uphold DEIA Values
OBSSR is committed to enhancing DEIA consistent with the NIH-Wide Strategic Plan for DEIA both within the behavioral and social sciences and across the biomedical enterprise at NIH, and to demonstrating this commitment with transparency and accountability.
Diversity within research teams and equitable participation in research are essential for OBSSR to meet its scientific priorities. Supporting investigative teams with a breadth of social and cultural influences and experiences is likely to expand and diversify the research topics within the overall basic BSSR portfolio.
Operational Priority 2: Build Equitable Partnerships and Collaborations
OBSSR is committed to building equitable partnerships and collaborations with NIH staff, federal agencies, and external partners to accelerate translation of BSSR evidence into programs, practice, and policies. To foster team science and enhance multidisciplinary integration, OBSSR strategically provides co-funding support for NIH-wide initiatives that encompass OBSSR priorities and intersect with the interests of multiple NIH ICOs.
Operational Priority 3: Enhance Communication among Scientists and With the Public
Over the next 5 years, OBSSR will reach out to new and more diverse audiences. This includes enhancing our existing content to make it more accessible and plain language, as well as creating new, impactful, and innovative content that highlights the importance of BSSR and communicates recent and exciting research to both the research community and the public.
31 Center Drive, Building 31, Room B1C19 Bethesda, MD 20892
Email: [email protected]
Phone: 301-402-1146
NIH Virtual Tour
- News & Highlights
- Publications and Documents
- Education in C/T Science
- Browse Our Courses
- C/T Research Academy
- K12 Investigator Training
- Harvard Catalyst On-Demand
- Translational Innovator
- SMART IRB Reliance Request
- Biostatistics Consulting
- Regulatory Support
- Pilot Funding
- Informatics Program
- Community Engagement
- Diversity Inclusion
- Research Enrollment and Diversity
- Harvard Catalyst Profiles

News & Highlights
Topics: Clinical & Translational Research , Five Questions
How to Accelerate Clinical Research
Five questions with michelle beck and yemi talabi-oates on making connections..

At Brigham and Women’s Hospital (BWH), oncologists who’ve been doing gene therapy trials for a decade are teaming up with researchers in other fields to apply their knowledge to the new wave of non-oncology cell and gene therapy studies and avoid recreating the wheel.
And at Beth Israel Deaconess Medical Center (BIDMC), one researcher’s challenges enrolling Black patients for a diet intervention study led to the development of a satellite center at a community-based clinic, which other researchers are exploring for their own studies.
These are among the ways Harvard Catalyst’s Connector sites facilitate and accelerate clinical/translational research across the hospitals affiliated with Harvard Medical School (HMS). Connector links investigators to the medical centers’ sprawling clinical research enterprises and troubleshoots research bottlenecks. (Boston Children’s Hospital and Mass General Hospital also have Connector sites.)
Need a research problem solved?
The premise behind Connector is that clinical research can be challenging, especially in complex academic healthcare environments. Individual scientists can’t do it alone. Connector sites help researchers get science done right–hopefully the first time–while maintaining the highest standards for patient protection, regulatory compliance, and quality.
Connector draws upon the collective expertise of HMS-affiliated institutions to guide studies through the lifecycle of science. For investigators struggling to recruit or wrestling with logistics, the programs offer a kind of life-raft of resources and support, big or small.
As the administrative directors of Connector sites at BIDMC and BWH, respectively, Michelle Beck and Yemi Talabi-Oates are like the Ghostbusters of translational research: They are “who you gonna call” when you’ve got a research problem to solve.
We caught up with them both in one Zoom to find out what they wish investigators knew about Connector.
What’s your cocktail-party summary of what Connector is?
MB: A cocktail-party summary is hard because Connector does a bit of everything, depending on what the investigator wants. Connector sites are really good at figuring out how to set studies up. Our special sauce, so to speak, is the experience in knowing how to make things work.
“Connector sites are really good at figuring out how to set studies up. Our special sauce, so to speak, is the experience in knowing how to make things work.”
At BIDMC, Connector encompasses our Clinical Research Center, a full-service operation providing research coordination and personnel for the lifecycle of a study, including recruitment. We provide laboratory, dietary, specialized nursing–because research nursing is a little different than clinical care nursing–and all affiliated services for inpatients and outpatients in our own research space as well as in other locations.
We help research teams get the tools and resources they need in other parts of BIDMC or across the network of HMS-affiliated hospitals. We might refer them elsewhere or integrate their research into our portfolio.
YTO: Brigham’s version of the Connector is the Center for Clinical Investigation (CCI). We call ourselves the home of clinical research. We are the first stop if an investigator needs help or wants to learn how to implement clinical and translational research studies.
Investigators can use any or all of our resources, from something small to running the whole study. We can connect them with potential collaborators or just find somebody to read an EKG, if that’s what’s needed.
We have clinical space dedicated to research so we can accommodate patient visits. But just as importantly, investigators have access to vital services that are not patient-facing, such as data management, research coordination, and biostatistics support.
What do you wish investigators knew about Connector?
MB: People sometimes think that Connector is the CRC. While it is at some level, it’s also much more. Our strength lies in the collective experience of the many people who have already figured out how to design and conduct high-quality studies, who understand the steps for getting from point A to point B.
Our program director regularly meets with investigators to provide feedback on their grant applications, offer advice on how to find funding, or connect them with mentors.
All of the Connector sites have a role called a navigator. BIDMC navigators are experts in regulatory and operations. Depending on when they’re brought in, they can point investigators to resources or work through specific aspects of their study that might be challenging.
YTO: What I find with investigators is they don’t know what they don’t know. They may come to us with one question and not realize how many other things need to be considered before we can address that one question. Having that dialogue as early in the process as possible will help the investigator in the end.
Connector lets investigators tap the experience of a diverse clinical research team, whether it’s the nurses on the floor or a physician-investigator who’s done this before. It’s about knowing your patient population and what works with recruiting, right down to which time of day is easier for patients. It’s helping avoid the pitfalls that may come with being a newbie to research.
“Connector lets investigators tap the experience of a diverse clinical research team, whether it’s the nurses on the floor or a physician-investigator who’s done this before. It’s helping avoid the pitfalls that may come with being a newbie to research.”
One of the things I often say to early-career investigators or those testing a really novel idea is if you’re going to fail, fail fast. It’s okay to fail because you can use what you learn to make the next study better.
Give us an example of something you’re engaged in right now that illustrates how the Connector sites work.
YTO: One of our big pushes right now is to help investigators in the non-oncology space who are interested in conducting cell and gene therapy studies. We don’t want to recreate the wheel, so we’re connecting them to oncology physicians who have been doing these studies for a while. We’re bringing together players who aren’t otherwise talking to one another to figure out how current systems might be adapted for studies outside of oncology.
MB: We have a general medicine investigator who is running a diet intervention study for hypertension, focusing on enrolling Black Boston residents in areas with ‘food deserts’ –areas with grocery store scarcity. This investigator met with our Connector team early in his grant planning process. His studies are now funded, and he’s running them through our main Clinical Research Center at the Boston campus. But he is having a really hard time meeting his recruitment goals, so we are working with him to set up research support at our Bowdoin Street clinic.
Because of that collaboration, the clinic is being developed as a CRC satellite with a focus on community-engaged research that allows the local community to provide input on the studies conducted there. We are now working with other clinical groups to expand research at the site.
So by addressing a recruitment problem one investigator was having, we’re establishing a resource that other investigators can access to bring research to our community and potentially improve participant diversity in their studies.
Connector’s goal is to accelerate translational science, in line with the mission of Harvard Catalyst and other clinical and translational science programs funded by the National Center for Clinical and Translational Science (NCATS). From your perspective, is clinical research efficient enough?
MB: I think sometimes we have unrealistic expectations about efficiency. I honestly believe that we make things very efficient in our programs and within our institutions, but I don’t think clinical research itself is efficient, through no fault of our own.
It’s really difficult to run a study, especially if you think about the whole lifecycle. For an industry-initiated clinical trial for example, a sponsor wants you to have a budget, contract, IRB approval, and be ready to enroll your first patient within 60 days of being selected as a site, which is crazy. It’s not impossible but it’s highly unlikely, with so many steps and the complexity of various studies.
If you move too fast, you risk making mistakes. If you don’t think carefully about what’s involved in a study from a participant’s perspective, for example, you might meet your recruitment goals right away but have 60% who don’t meet inclusion/exclusion criteria at screening and 20% who didn’t realize they’d have to come in every day for the next six weeks. Those kinds of circumstances can be largely avoided with the right planning.
YTO: We definitely take some time on the front end to get all the information and tweak things where necessary to make sure the patient’s experience and the study team’s experience is positive and efficient. We want to make sure it’s done right, and that might take a little longer.
In the end I’d rather be confident that patients are safe and the data is accurate because we thought these things through up-front. That’s the time to do it, not when the patient shows up for the visit or the investigator sits down to write that paper with flawed data.
How does Connector affect the experience of patients involved in clinical research and why does that matter?
MB: Using a Clinical Research Center is hugely beneficial from the participant perspective. We had a study many years ago in which two people came in every month for seven years for the same study. They got to know each other, and they knew all our staff; they would even bake banana bread for the research center staff. I think if patients get to know the research center, that’s another mechanism for patient retention, particularly for longitudinal studies.
YTO: Agreed. Our nurses and clinical staff on the floor definitely make connections with the research participants, and they often learn something that could be valuable to the study team. Having that connection is key.
It’s not just the study team; it’s every member of the staff. It’s the person who smiles when they walk in the door, or the one who knows about their child or their cat and asks about it. That matters when you want a patient to come back again and again into the next month or year.
Sign up to receive our newsletter: courses, funding, events, and resources.
- BCC Library
- Mission Statement
- Distance Learning Students
- BIP Students
- Faculty/Staff
- Community Patrons
- Code of Conduct
- Computers & Printing
- Interlibrary Loan
- In the Library
- Search the Catalog
- A-Z Database List
- E-Journal Portal
- Student Art
- Dolphin Die-Jest
- Popular Forms
- Book Review Submissions
- Comments/Complaints
- Interlibrary Loan Requests
- Library Instruction Requests
- Research Appointments
- Room Reservations
- Suggest Library Purchases
- Continuing Education
Service Alert

NUR 211 - Parrella - Clinical Research Paper: Plan Your Search
- Quick Start
- Guide Concept Map
- EBP Levels of Evidence
- Plan Your Search
- Find Articles
- Write & Cite
- Ask a Librarian
The format:
P – Patient, problem or population I – Intervention C – Comparison, control or comparator O – Outcome(s) (e.g. pain, fatigue, nausea, infections, death)
- PICO tutorial | Arizona State University Library An interactive tutorial that introduces the PICO format and how it can be used effectively to formulate a research question.

Source: Learning Resources Center, Logan University
CINHAL Subject Headings
- CINAHL Plus with Full Text Use "CINAHL Subject Headings" feature to build a keyword search with its indexing terms.
MeSH on Demand
- MeSH on Demand | National Library of Medicine Use this AI powered search box to generate National Library of Medicine MeSH subject headings (controlled vocabulary) for your search and to find full-text PubMed articles or to use articles for citation chaining.
PICO Worksheet (PDF)
- PICO Worksheet Use this printable worksheet to plan your search strategy using PICO and to select relevant databases for your search.
Problem/Solution Prompt & PICO (Basic)
Clinical Research Question: Among intensive care (or acute or critical care) nurses, will mindfulness-based interventions and increased physical activity (or exercise interventions) decrease burnout syndrome (professional burnout) and increase job satisfaction (retention)?

PICO Tutorials & Resources
- Search Development Strategy: PICO & PCC | The University of Alberta
- << Previous: EBP Levels of Evidence
- Next: Find Books >>
- Last Updated: Aug 20, 2024 10:29 AM
- URL: https://brunswickcc.libguides.com/nur211_parrella_clinicalresearchpaper
More From Forbes
Representation in clinical research calls for a new approach.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Rohit Nambisan is CEO and co-founder at Lokavant , a clinical trial intelligence platform company.
Increasing clinical trial participant representation in clinical research is critical to developing new medicines that are safe and effective for patients in need. However, historical clinical research, from which the industry draws insights for future studies, often lacks diverse data, perpetuating imbalances and leaving key patient populations disenfranchised.
It doesn’t have to be this way—provided our industry is willing to rethink current approaches, implement more community or patient-centric outreach programs and invest in smarter use of new technologies.
Understanding Disease Impact: The First Step To Greater Representation
The FDA is urging the industry to enhance diversity and inclusion in clinical trials, issuing guidance in 2022 on drug development approaches to facilitate these goals. Recommendations include making trials more accessible with reduced visit frequencies, flexible scheduling and digital communication tools, alongside early engagement with patient advocacy groups to design appealing study protocols.
Identifying patients for clinical research is particularly challenging as drug developers target diseases affecting highly specialized patient groups. Current trials focus on developing therapies for patients with specific disease markers, genetic abnormalities or subsets of conditions to demonstrate therapy efficacy.
Moderna ran into representation issues in 2020 while testing its Covid-19 vaccine. The company hired contractors to find 30,000 participants for its clinical trial, but these participants were mainly white. At the time, Covid-19 infected Black Americans at three times the rate of white Americans. To its credit, Moderna paused the trial until it had boosted the Black patient participation to nearly 10% and the Hispanic participation to around 20%—a more representative patient pool.
Real-world data (RWD), beyond just historical clinical research data, can be leveraged to identify patients who meet specific eligibility criteria for new studies. RWD consists of datasets representing care delivery episodes and longitudinal patient interactions among groups of patients with the same disease.
Recent technologies enable anonymization, allowing patients or patient groups to be uniquely tracked through their health journeys without revealing their identities. Additionally, RWD can be used to identify and meet the diversity, equity and inclusion (DEI) goals that the FDA requires for new studies.
How Researchers Can Avoid Misrepresentation In Clinical Trials
It’s not enough to simply identify clinical trial sites in areas with more diverse populations. These sites might not have previous experience running clinical trials, or they might encounter challenges in recruiting eligible patients. That’s why the clinical research industry must drive awareness of a recruiting clinical trial for both doctors and their patients while working to overcome inherent mistrust of the healthcare industry.
The general perception of pharmaceutical companies still lags behind the perception of companies in other industries. According to the 2024 Edelman Trust Barometer , "Scientists are still trusted—but increasingly subject to public scrutiny. To build trust in expert recommendations, explain the research, engage in dialogue, and harness peer voices as advocates."
Some patients, especially those living with rare diseases, have had to become strong advocates for clinical research to fuel the pursuit of new therapies. Many have formed organizations, raised funds, lobbied regulators and even directed their own clinical trials .
Research organizations should collaborate closely with advocacy groups to mitigate distrust and misinformation among patients, aligning with FDA regulations to strengthen alliances and meet diversity goals. Expanding clinical trials internationally offers another avenue to enhance diversity, leveraging countries with diverse patient populations and addressing globally prevalent diseases and conditions effectively.
Big Data: A Blessing And A Curse
Researchers can also consider new ways to improve representation using systematic data analysis. Today, there are ever-growing volumes of data available to help trial sponsors identify specific patient populations and meet DEI goals aligned with FDA-requested diversity action plans. However, managing all this data is difficult.
According to a 2021 study by Tufts University , "Phase III clinical trials currently generate an average of 3.6 million data points, three times the data collected by late stage trials 10 years ago." Lokavant’s internal analysis suggests that these clinical trial datasets will skyrocket to seven times that of 2011 by 2030.
Today’s avalanche of data presents both opportunities and challenges. Effectively analyzing increasingly disparate data types and volumes is crucial, especially with the FDA’s new DEI requirements for clinical trials, which elevate the importance and complexity of data analysis.
Small to midsize biopharma companies may find compliance with these FDA rules cost-prohibitive, potentially hindering innovation. Leveraging advanced data approaches, such as technologies that centralize various data types, can significantly enhance these efforts by predicting challenges like inadequate patient recruitment within feasible timelines.
A Prescription For More Representative Clinical Trials
Despite the issues and complexity, industry leaders can make clinical trials more patient-friendly and increase representation. Here are my recommendations:
• Patient Feedback: This informs trial improvements. For instance, decentralized data collection, popularized during the pandemic, eases participant burden, enhancing recruitment and retention.
• Community Outreach: Implement community outreach programs to leverage local networks among diverse patient groups. The Yale School of Medicine’s Cultural Ambassadors program , which links investigators directly to resources in the community for outreach and education, is a great example.
• Unbiased Data: Leverage technologies that enable access to unbiased datasets. While technology can’t sway individual perceptions, preconceived notions, mistrust and biases, it can do a lot to improve how we design clinical trials and monitor them in real time.
• Measuring Twice, And Cutting Once: When designing clinical trials, use data-driven feasibility to target optimal trial sites and geographies for better population representation.
The aforementioned patient-centric approaches can make it easier for patients across diverse populations to participate in clinical research, thereby creating a formula to end decades of failed attempts (registration required) to boost trial representation, as well as helping drug developers comply with FDA diversity requirements.
The desired end result will be the rapid development of safe and efficacious medicines that benefit those most in need.
Forbes Technology Council is an invitation-only community for world-class CIOs, CTOs and technology executives. Do I qualify?

- Editorial Standards
- Reprints & Permissions
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Strategic Management in Healthcare: A Call for Long-Term and Systems-Thinking in an Uncertain System
Associated data.
Data sharing not applicable.
Strategic management is becoming increasingly important for sustainable management in healthcare. The reasons for this can be seen in the increasing complexity, dynamics and uncertainty of the system’s regimes and the resulting need for strategic thinking in a long-term period. The scientific discussion of this issue is the aim of the present analytical framework. The starting point is the definition of the term strategic management itself, followed by a reflection on the requirements resulting from the changes in the political, social and economic value systems of our post-industrial society. In this context, Dynaxity Zone III is used to explain the long-term perspective, the high levels of complexity and uncertainty and the responsibility of strategic management as important parameters. For a practical illustration, we demonstrate two selected applications (German hospital financing systems and development process of implants) and how the implementation of strategic management in the health care system shows success.
1. Introduction
“Strategy” and “strategic management” have become buzzwords that are frequently used in the practice and theory of healthcare. However, the terms are not as simple as they might seem, and in reality, many managers are still micromanaging without a strategic perspective. Consequently, it is worthwhile to unfold the meaning of the terms and to analyse their relevance in healthcare.
The term “strategy“ stems from ancient Greek word “στρατηγός“ (strategos) meaning “general” or “leader of an army”. Thus, the original meaning of strategy is the theory or study of warfare and everything a good leader of an army should know. Carl von Clausewitz (1780–1831) developed in his famous book “ Vom Kriege ” ( About War) the first (European) theory of strategy distinguishing between tactics and strategy [ 1 ]. The first term describes the organization and fighting of forces on or near the battlefield, while the latter term goes far beyond that and tries to utilize different instruments for the final objective of winning the war. This not only includes battles but also withdrawals, alliances, negotiations and circumventions. V. Clausewitz was a Prussian officer serving the Russian Czar during the Russian Campaign (1812–1813). He realized that the French army won all battles but finally lost the war. The strategy of Prince Mikhail Illarionovich Golenishchev-Kutuzov (1745–1813) was to withdraw and even avoid battles—an approach of warfare that was unusual at that time and even made some to accuse him as coward. His credo “We must win the war—not the battle” strongly influences the strategic thinking of v. Clausewitz in his later years as the director of the Prussian “Kriegsakademie” (college of war) and enfolded his theory of strategy.
For v. Clausewitz, strategy has four dimensions that are relevant not only for warfare but are widely applied in management today such that “ Vom Kriege ” is mandatory reading in many business schools until today. These dimensions are as follows [ 2 , 3 ]:
- Long-term: Strategy always focusses on the long-term consequences of actions. The manager—as the commander-in-chief—should pay more attention to the final result than to the intermediate gains.
- Strategic apex: Strategy is the main responsibility of the top-leaders as it always covers and affects the entire organization. There is no “middle-management strategy”.
- Complexity: As the strategy covers the entire organization and long-term consequences, many different elements and dimensions are involved, i.e., strategy has to deal with a high degree of complexity.
- Uncertainty: The long-term consequences of actions are highly uncertain.
The terms “complexity” and “uncertainty” are crucial for strategy and require more explanation. “Complexity” stems from Latin “cum plectrum“, meaning connected, interwoven or interdependent. Thus, a system is not complex because it consists of many similar elements, but because the elements are different and have a high number of relations between them. These relations are frequently non-linear or even non-monotonous. Consequently, complex systems cannot be described with all their behavior even if all information on each single component exists [ 4 ].
Uncertainty means that the conditions of the environment and system behavior are not known and/or their transitions are subject to certain probabilities [ 5 ]. The longer the distance between the point of planning and the point of action, the higher the degree of uncertainty. Some uncertainty (e.g., epidemics and crop failure) is external and cannot be influenced (“Act of God”); other uncertainty includes the consequence of many small decisions and events, which add up and result in chaotic system behavior. Frequently, this kind of uncertainty exists because we have a rational opponent or antagonist. This is the field of strategy seeking to achieve one’s own objectives while expecting countermeasures of the opponent but also building alliances with protagonists [ 6 ].
Consequently, a strategy is a long-term plan of action of the strategic apex of an organization that analyses the complexity and uncertainty of the system and makes decision under the consideration of all potential stakeholders [ 7 ]. For a business unit, we have to distinguish the following:
- Domaine: What is our business field, i.e., with what products to do want to serve which group of customers with which needs?
- Competition: How do we want to set ourselves apart from competitors (quality leaders, price leaders and niche)?
- Competence: What is our core competence and how can we develop it (resources and potentials)?
- Alliance: With whom do we want to achieve our goals and how strongly do we cooperate?
It was frequently stated that operational management means “to do things right”, while strategic management means, “to do the right things” [ 8 ]. With v. Clausewitz, we could argue that it is correct but insufficient. Strategic management means “to do the right things right” by focusing on the long-term consequences of our actions in an environment of uncertainty and complexity. While we develop strategies, we do not know all the parameters, we expect new interdependencies to arise and we have to deal with stochastics and decide on alliances and competition. Strategy is the supreme discipline of management.
Figure 1 shows the strategic management process. The starting point always involves strategic objectives including the vision and mission of the enterprise. This is the domain of business ethics, i.e., strategic management without ethical reflection on the value and resulting objectives is infeasible. Based on these objectives, we analyse the environment and the enterprise for chances and risk with respect to strengths and weaknesses. This includes the development of a strategy or a set of strategies. Based on the objectives, the strategic manager selects a strategic program and implements it. In principle, the strategic management process is similar to a general management process, but the time-frame, the degree of uncertainty, the relevance of the decisions and the number of sub-units of the environment and the enterprise involved are much higher [ 7 ].

Strategic Management Process. Source own, based on [ 7 ].
These days, many healthcare services are more influenced by etatism than many other fields of business administration. The traditional time-horizon of healthcare is the annual budget provided by governments or parastatals (e.g., social health insurances) [ 9 ]. The main purpose of the “traditional” administration of healthcare services is the compliance with laws and regulations, while the efficiency and long-term development of potentials are still less focused upon. Even if the first efforts towards a strategic approach have already been made by larger healthcare systems and individual profit-oriented institutions, strategic thinking and management have not yet been sufficiently recognized and implemented in most traditional healthcare systems and non-profit organisations. In this paper, we argue that more long-term systems thinking on the strategic apex with consideration of dynamics, complexity and uncertainty are crucial for the healthcare system.
For this purpose, the next two sections discuss the characteristics of healthcare systems in the post-industrial era. Afterwards, we analyse the instruments and personal characteristics required to implement successful strategic management in the healthcare field. This knowledge is applied to examples, namely hospital financing in Germany and research and development of implants. The paper closes with some conclusions on how strategic thinking and management can contribute to the health and wellbeing of human beings.
2. Dynaxity
There seems to be general agreement that the last decades have witnessed tremendous changes in political, social, economic and value systems of our societies. The development from the industrial era to the dominance of service industries, globalisation and individualisation has frequently been discussed [ 10 ], but their impact on healthcare systems is insufficiently reflected. Rieckmann introduced the term “Dynaxity” as an artificial construct to describe the economy and society of the new millennium with the three characteristics: dynamics, complexity and uncertainty [ 11 , 12 ]. In this section, we will unfold these dimensions of Dynaxity and analyse their relevance for healthcare systems and management.
The term Dynaxity describes the dynamics, complexity and uncertainty of a system. Every an open system has a tendency to restore its steady-state-equilibrium and avoid changes because any alteration requires energy and induces uncertainty; i.e., open systems are usually homeostatic [ 13 ]. Only when the differences between goals and outcomes of the system are so strong that the formal and material structure cannot be maintained is the system has to react and adjust its structure. Otherwise, homeostasis will lead to the extinction of the system. Economic systems are constantly under the pressure to change as the environment changes frequently. Under the pressure of change, they will only survive if they can expand beyond their original limitations.
2.1. Transformation
Originally, the system is in a steady-state equilibrium. It fulfils its function in its environment and is able to absorb smaller internal or external perturbations (synchronic systems regime). If the perturbations grow so strongly that they cannot be absorbed any longer within the existing structures, the system begins to fluctuate until it reaches a bifurcation point where it is obvious that the system will never be the same again. In most cases, the system will find a new equilibrium, which is adjusted relative to the new environment and usually on a higher energy level ( Figure 2 ).

Transformation into a new systems regime. Source own, based on [ 13 ].
Changes in the environment are first absorbed by the microstructure (e.g., personnel, customers). Only if the perturbations are rather strong such that the microstructure cannot handle it will the meso structure (entire system) become involved. Moreover, the mesostructure will be passed onto the macrostructure, i.e., the economic or political system, only if it cannot absorb the fluctuations. A stable mesostructure can absorb quite an amount of pressure, but if the necessary changes are blocked by the macrostructure, the mesostructure might become inflexible or even fragile.
The development of new structures and functions of systems require a steady flow of energy. Ecological systems are finally based on the flow of energy from the sun, but social systems can utilize the creativity of human beings as the ultimate source of energy to adjust the systems. With creativity, humans develop innovations to respond to changes of the environment and survive them. Thus, innovations are the foundation of the survival of open systems, and their evolution is the condition for survival. However, innovations are not only the solution for problems but also the cause of perturbations. In a dynamic economy, an innovation will prosper the innovative enterprise but challenge other organisations based on old standard technology. As Schumpeter showed more than a century ago, competition usually means “creative destruction” [ 14 ]. One enterprise solves its challenges by an innovation, and others are driven in a crisis by exactly this innovation. They require further creativity and innovation to respond to this crisis and develop another innovation, which will then become the new standards again and cause another crisis in other enterprises.
2.2. Zones of Dynaxity
The sequence of synchronic and diachronic system regimes is not only accompanied by an increase in energy but also an increase in complexity and dynamics. Depending on the degree of complexity and dynamics, different zones of Dynaxity (I-IV) can be derived [ 15 ]. In zone I, the system consists only of a few elements and the number of interdependencies and relations between these elements is small. The number of relevant changes within a time interval (dynamics) is rather limited at well; i.e., the system can be called static. Consequently, almost all elements, their behaviour and the interdependencies are well-known; there is little uncertainty within the system. Zone I is typical for pre-industrial organisations, but even today, some private practitioners work in zone I with a small number of staff, clear hierarchies, strict control of processes and a stabile function within the village where they are located. According to Mintzberg, this is a simple structure [ 16 ].
If complexity and/or dynamics increase, simple structures will be insufficient for survival in an altered environment. Consequently, zone II is an industrial era with big organisations comprising many hierarchical levels. These organisations follow strict rules of the division of labour, leading to efficiency gains that are previously unknown. However, they are also slow because the flow of information through the different layers of hierarchy takes some time. Thus, these technocracies and bureaucracies [ 16 ] are inadequate if the dynamics or complexity grow even stronger.
The post-industrial era is characterised by very high complexity and dynamics leading to high uncertainty. The “dinosaur” organisations with long information pathways cannot adjust sufficiently rapid to survive the ongoing changes. Instead, organisations must be networks with a tremendous number of interrelations, institutional memory and intrinsic motivation of co-workers who are able and willing to sense changes of the environment early, adapt the structure of the network accordingly and develop innovations to keep the original function of the enterprise [ 11 ].
Finally, if dynamics and complexity increase even further, uncertainty will grow to a degree that makes any prediction or separation of diachronic and synchronic phases impossible. Rieckmann calls this system “Chaos” (χάος) in the sense of a state of complete disorder [ 12 ]. Proactive management becomes impossible as there is no reliable information on the interdependencies and behaviour of the multitude of different elements of the system with a complete tohu wa-bohu (( תֹהוּ וָבֹהוּ , Genesis 1:2) without any predictability. Figure 3 shows the four zones of Dynaxity.

Zones of Dynaxity. Source own, based on [ 12 ].
The zones I-IV can also be interpreted as development pathways of systems regimes, as shown in Figure 4 [ 9 ]. In a system of zone I, the systems regime changes only rarely, i.e., the synchronic phase has a duration of at least one generation. In zone II, the synchronic phases are shorter than in zone I, but they are long enough to permit a complete stabilisation. Traditional change management includes the final stage of “freezing” which makes only sense if the period of stability is sufficiently long to establish stabile meta-structures with organisational designs, regulations and hierarchies [ 17 ]. In zone III, however, stabile phases are so short that no steady-state equilibrium is possible at all. Instead of freezing the organisational structure at the end of the diachronic systems regime, a new and fundamental perturbation waits for the system. Consequently, no fixed rules can be developed and implemented, but ad hoc decisions and structures are required to deal with a steady flow of fundamental changes. However, the decision in a highly complex environment needs a high density of information requiring turbo networks without hierarchies and with a broad span of interaction instead of slow hierarchies. The chaotic system, finally, does not allow distinguishing phases or predicting the pathways of development.

Dynaxity and Systems Regime. Source own, based on [ 9 ].
For the longest period of time in human history, societies and economies persisted in zone I. Most severe perturbations were external shocks such as famines, epidemics or wars, which could have disastrous consequences such as the medieval plague epidemic (1346–1353) that killed about 1/3 of the population in Europe. For the individual and for enterprises, these shocks were “act of Gods”; i.e., they could not proactively take action or make fundamental changes as they did not have the knowledge how to alter their fate. After the external shock was no longer a threat, life continued—in principle—unchanged, with only a few innovations of limited relevance for daily life within a lifetime. Innovations were seen for the longest period by human beings as something negative—a swear word challenging the (God-given) order of the society. For instance, Wilhelm von Conches (1080–1154) expressed his own mission with the words “sumus relatores et expositores veterum, non inventores novorum” [ 18 ] (we are the mediators and explainers of the old, not the inventors of something new). The technology and regulations of the past were right—innovations were seen with suspicion.
Several basic changes increased the speed of economies and societies and opened the doors for industrial revolution, bringing unknown dynamics and complexity until then. At least for Europe, we can state that the reformation and the age of enlightenment together with the French revolution and liberalism (for instance, Adam Smith) made it possible for innovations to become the driving force of development. “Creative destruction” started and constantly increased the speed of changes [ 19 ].

2.3. Uncertainty
In zone III, we face all forms of uncertainty: We do not know which elements of the system are relevant to us, because while we observe the system, it is changing dramatically with new elements coming up and others being left out. We do not know the interdependencies between these elements as the system has become so complex that it cannot be described in its system behaviour even if we can describe each element. Moreover, all behaviours of the elements and the system are stochastic processes with fairly unknown probabilities. There is even a risk that the system becomes chaotic where no trends can be determined and even minor changes of seemingly irrelevant parameters have major impact on the entire system.
A major cause of uncertainty is the complex system of side effects, feedback effects and knock-on effects ( Figure 5 ). Any action has a primary effect, i.e., an intended effect of a parameter A at the time of intervention. At the same time, the action has a side effect on another parameter B at the same time as the action but without any intentions. This change of parameter B might have an impact on parameter A, which can be delayed, accelerated or decelerated and is called feedback effect. Furthermore, a change of parameter B can have an impact on parameter C (knock-on effect), which will itself induce side effects, feedback effects and other knock-on effects resulting in a chain reaction, which is highly uncertain.

Side, feedback and knock-on effects. Source own, based on [ 6 ].
Summarizing these findings, we can state that the post-industrial society and economy are in Dynaxity zone III characterised by high dynamics and complexity resulting in high uncertainty without stable phases. Change is the “new normal”, and peaceful stability is the exemption. The system cannot be described or analysed to the full extent as many new elements and interdependencies develop and any action has an impact on many elements now and in future. These characteristics constitute major challenges to our ability to design systems and make meaningful decisions because our brains are not designed for systems with these characteristics.
Dörner demonstrates that the human capability to understand complex, dynamic and stochastic systems and make rational decisions within such systems is limited. Without referring to Dynaxity or the post-industrial age, Dörner shows that human beings have, in particular, problems in understanding the dynamics of exponential developments. The human brain thinks linearly, but nature grows exponentially. He shows that human brains are overburdened with increasing growth rates and systematically under-estimate the increasing speed of exponential processes. In addition, uncertainty with incomplete information (because of complexity) leads to false hypotheses about causal connections. The more complex, dynamic and uncertain a decision situation is, the more likely human beings make poor decisions, and the overburdening grows itself exponentially with the size of these three parameters.
Consequently, management in zone III is bound to fail unless it explicitly considers dynamics, complexity and uncertainty. Traditional management was short-term, comprised rather limited sub-systems and ignored uncertainty. However, the more intensive zone III becomes, the less it will be functional. Instead, managers have to develop a strategic mindset with explicit considerations of these three dimensions, the appropriate instruments for strategic leadership and a strategic leadership style with a strategic leadership personality.
3. Management in the of Post-Industrial Era
Management in Dynaxity zone III must be different from management in zone II. In principle, management in zone II focused on operational management, but during the short diachronic phases, the elements of strategic management were added. During the fluctuations, the existing structures were broken-up (unfreezing) and new elements were designed so that the enterprise fits again with respect to the changed environment (moving). Afterwards, everything was fixed again (freezing) with the aspiration that this condition should last as long as possible. During the synchronic phase, strategic management was grossly neglected.
In zone III, there are no synchronic regimes; thus, change is an ongoing process without freezing. Consequently, managers have to perform strategic management permanently and not only during certain phases. Instead, they are constantly seeking for challenging changes of the environment and upcoming innovations, risks and potentials. Thus, strategic and operational management are not contradictory but have to be implemented simultaneously and have to be synchronised constantly. However, their instruments are quite different and this requires a completely new armamentarium of the manager.
Table 1 shows the differences between operational and strategic management. It is obvious that successful instruments and approaches of operational management are quite different from what is needed for strategic management. If the environment does not change strongly during a synchronic phase, the organisation can focus on short-term plans, leave decisions to middle- and lower-level management and limit the decision-field to a few alternatives. The main instrument here is managerial (cost) accounting, expressing business success in currency units. However, when the environment becomes turbulent, this approach is likely to fail. Adoption and adaption, changes and evolutionary jumps are required to survive in diachronic phases. Thus, accounting and focusing on finances are insufficient to conquer the future. Instead, potentials have to be developed in the end, and chances and risks as well as strengths and weaknesses have to be analyzed.
Operational and Strategic Management. Source own, adapted from [ 20 ].
| Operational Management | Strategic Management | |
|---|---|---|
| lower management level; resorts | strategic apex; entire enterprise; covering all resorts | |
| short-term | long-term | |
| Return-on-investment of existing business processes | Potentials of success | |
| payment and receipts, income and expenditure, cost and revenues | Chances and risks, strengths and weaknesses | |
| Reduce complexity and uncertainty; many details; dominance of administration; internal orientation; many unconnected plans; high commitment of a plan; inflexible systems; limited decision field | high complexity and uncertainty; poorly structured problems; strategic planning and control; comprehensive business models; limited commitment to plans; flexibility; broad decision field | |
| Profit, Solvency | Development of potentials of success through investment; management of change and systems development; search for new functions | |
| Profit- und Cost-Centers | strategic business units | |
| Accounting | Portfolio-analysis; causal loop diagrams, balanced score card, scenarios/simulation |
Typical instruments of strategic management are portfolio analyses, causal-loop diagrams and simulations/scenarios. A portfolio analysis is a visual presentation of the different products and their relevance for the achievement of the long-term targets. Based on the classic BCG-matrix [ 21 ], many portfolio analyses have been suggested for different purposes. For instance, Schellberg designed a portfolio matrix for nonprofit organizations distinguishing the dimensions of “ethical call” and “finance ability” [ 22 ] ( Figure 6 ). The first dimension describes the relevance of a service for the achievement of the target system of the non-profit organisations (NPO); i.e., each NPO has to decide whether a specific service is crucial for the achievement of the target system of the NPO or not. The second dimension analyses whether an NPO can breakeven at a given financing regime. The arrows indicate that many products start as touchstones (high ethical call, but deficit), move towards stars (high ethical call, profit) and end as goiters (low ethical call, deficit).

Portfolio Matrix of a non-profit organisation. Source: own, based on [ 22 ].
The portfolio analysis reduces complexity by developing norm strategies for the four fields. It also allows analyzing the life cycle of products and, thus, reducing the perceived dynamics and uncertainty. Thus, it is an appropriate instrument of strategic management.
Causal loop diagrams are a visualisation of causes, consequences and interdependencies. Figure 7 shows a causal loop diagram for the infectious cycle of malaria [ 23 ]. An infected anopheles bites a non-infected human who might become infectious after some time. If another anopheles bites this infectious human, it can be infected and become—after some delay—infectious again so that the cycle starts anew. The autocatalytic cycle is the basis for exponential growth, which is very difficult to understand for human brains. However, the causal loop diagram clearly demonstrates the interdependencies between the variables. Thus, it reduces complexity and, consequently, uncertainty.

Causal Loop Diagram of Malaria. Source own, based on [ 24 ].
The balanced score card (BSC) can also be described as a causal loop diagram as it connects the different dimensions of strategic business performance [ 25 ]. While operational management frequently focusses on one performance dimension (usually profit), a BSC includes other dimensions (such as potentials, customer satisfaction, etc.) and shows their interdependencies. This approach reduces complexity and uncertainty by indicating the respective causalities of strategic success.
Finally, the degree of uncertainty grows exponentially with the distance between the day of planning and the day of action, i.e., the higher the time horizon, the higher the uncertainty. Consequently, strategic planning is planning under uncertainty with many different alternatives that can occur. This is reflected by scenarios or simulations. Uncertainty can have different dimensions, i.e., we can have uncertainty concerning parameters (e.g., medical infectivity of a virus), uncertainty about certain structures (e.g., natural reservoir of a virus) and uncertainty concerning processes (e.g., impact of an intervention program on incidence) [ 26 , 27 ]. Consequently, we simulate the impact of changes of parameters, structures and equations on the long-term results of a system or an intervention in the sense of “What-if?” Furthermore, we analyse which parameters, structures and processes are necessary for achieving a certain result in the sense of “How-to-achieve?” Finally, we develop scenarios of constellations of parameters, structures and relationship, which are “worst”, “likely” or “best” in order to determine a corridor of potential developments of outputs. Thus, scenarios and simulations are instruments for reducing uncertainty and—partly—dynamics by developing a sensation of future realities and their probabilities.
In summary, we can state that strategic management is different from operational management. Strategic management has to deal with dynamics, complexity and uncertainty and requires a different set of instruments. However, strategic management is not primarily a question of a toolbox with strategic instruments. Instead, we see our organizations and the environment with a paradigm. This mindset must be future-oriented, risk-taking, cooperative and open for innovations. The strategic manager is constantly seeking new opportunities to serve the function of his organization better.
Henning and Rieckmann introduced the term “dynaxibility” to express the ability of an individual or an organisation to deal with Dynaxity [ 28 ]. In zone III—in terms of their conclusions—technical or hierarchical solutions are insufficient for achieving organisational objectives. Instead, the networks have to be viewed as “living systems” with human beings with personalities that go beyond the traditional assumption of the agent of production “labour”. Co-workers in zone III are seen as “complex men” [ 7 ] with their own feelings, aspirations, likes and dislikes. They cannot be fully “managed” but require identifying a valuable goal, sense the meaning of their work and have a chance of personal development [ 29 ]. Table 2 shows some characteristics of effective leaders in zone II. We allocate the terms given by Rieckmann to the characteristics of zone III. It becomes obvious that the characteristics of a “good leader” in Dynaxity zone III focus on the ability to deal with dynamics, complexity, uncertainty and people. It is also obvious that no single leader can have all these abilities; i.e., management in zone III has a tendency to result in team-effort.
Characteristics of high dynaxibility. The allocations to the terms dynamics, complexity, uncertainty and people-orientation are marked with an X. Source: own, based on [ 11 , 12 ].
| Characteristics | Dynamics | Complexity | Uncertainty | People-Orientation |
|---|---|---|---|---|
| Acceptance of permanent changes | X | |||
| Ability to thinking in networks and processes | X | |||
| Multi-cultural sensitivity | X | X | ||
| Creativity | X | X | X | X |
| Rapidness, speed | X | |||
| Ability to communicate effectively | X | |||
| Acceptance of uncertainty | X | |||
| Generalists | X | |||
| Stress tolerant | X | X | X | |
| Ability to reflect, perceive meaning | X | X | ||
| Abstract thinking | X | X | ||
| Ability to deal with conflicts | X | |||
| Ability to work and lead in teams | X | |||
| Understanding group processes | X | |||
| Thinking in and living with interdependencies | X | |||
| Ability to work without hierarchies | X | X | ||
| Ability to learn and teach | X | X | ||
| Willingness to share knowledge | X | X | ||
| Sensibility to framework conditions | X | X | X | X |
| Risk-taking | X | X | X | |
| Strong future orientation | X | X | X |
4. Applications
The healthcare sector of many countries is now in Dynaxity zone III. In this section, we will present different examples from the healthcare sector to underline our statements and show the impact of zone III for the management of healthcare services and systems as a call for more strategic management.
The first example follows the synchronic and diachronic phases of the pathways of German hospital financing and demonstrates the relevance of the Dynaxity model for this development. The second example provides a model of the development of innovative implants and, in particular, the need to reflect on the lifelong consequences of implants as the strategic dimension.
4.1. Hospital Financing in Germany
Figure 8 exhibits the phases of German hospital financing. We can distinguish five major phases [ 9 ]. Until 1936, hospital financing in Germany was almost free and did not have to follow any Governmental regulations. Health insurances funds negotiated rebates with the hospitals, which were based on daily rates and covered all costs (monistic financing). The system was functional for decades, but medical and social progress required more Government interferences. More and more services could be provided by hospitals and the costs exploded such that the national socialists interfered in the previously free hospital market and ordered a price stop. Hospital financing instruments (monistic and daily rate) remained unchanged, but the Government fixed the rules of calculating the rates. A consequence was that German hospitals could not follow international medical and technical developments. In 1948, the Government of Western Germany attempted to return to the original free system, but the prices exploded. Consequently, only six months later, the government interfered again and fixed the prices.

Phases of German hospital financing. Source own, based on [ 9 ].
During the first years after World War II, the prospering economy provided sufficient funds to finance hospitals (at least in Western Germany). However, the rapid technological progress of medicine as well as the first economic crisis after WWII in the 1960s placed pressure on the government to support hospitals financially. The solution was dual financing (1972), where the health insurance funds refund current expenditure while the government is responsible for funding the buildings, equipment and vehicles of hospitals irrespective of ownership. At that time, some people preferred returning to a government-free system, but dual financing strengthened the role of the government.
The innovative financing system was quite successful, but German hospitals remained quite inefficient in comparison to other countries. After reunification, Eastern German hospitals (which had had a budget-based hospital financing system since 1946) required tremendous funds to reach the Western German level such that the inefficiencies became a challenge. Consequently, policy makers searched for alternative financing regimes. Some wanted to return to the monistic system prior to 1972, but it was agreed that the system should remain dualistic but based on flat rates, which involved the so-called German Diagnosis Related Groups (G-DRG).
One consequence of this system was that the payment of the insurance schemes is a price that need to be paid and the hospitals decided how they could use this price to recover their costs. For different reasons, nurses became the piggy bank of hospitals; i.e., the number of nurses and their salaries declined in comparison to other cost items and staff categories. The result was a “nursing crisis”, which placed strong pressure on politicians. Some wanted to return to monistic financing, and others wanted to return to daily rates. The selected solution is a mixed financing regime where the cost of nursing is taken out of the G-DRG system and financed by a specific nursing budget while other recurrent costs are financed by flat rates. This system (called aG-DRG) was introduced in 2019 [ 30 ].
Based on Figure 8 , we can conclude that German hospital financing went through a number of synchronic and diachronic system regimes. The solution of the old crisis was frequently the seedling for the new crisis [ 31 ]; i.e., it is likely that the fifth phase is not the final endpoint but new phases will occur. During the five phases, the hospital financing system developed from Dynaxity zone I to zone III. The number of changes (expressed in major regulations for hospital financing) has steadily increased in the last 100 years. While there were hardly any major alterations in the first decades, there are currently several major changes per year. The dynamics has proceeded from static to turbulent.
At the same time, the system has become increasingly complex. Until the year 1983, hospitals produced only one single service unit, the bed day. From 1983 to 2003, hospitals (with some exceptions) were also financed by daily rates, but they were not calculated per bed day for the entire hospital, but for each department; e.g., a hospital with 10 departments had 10 different services. Since the introduction of G-DRGs as a compulsory financing system, hospitals have more services (year 2022), with almost 1300 different services. Thus, not only has the technology of medical services become increasingly complex but also the financing regime. Instead of having a one-product enterprise, we have a complex multi-product enterprise. There is no doubt that German hospital financing is in zone III and uncertainty with unexpected frequent substantial changes is a constant threat for hospital planning.
The introduction of G-DRGs was a major call for strategic management in German hospitals. While the annual budget was the pivotal unit in German hospital management before, DRGs forced management to think years ahead and to develop a production plan that allows fulfilling the function of the hospital and its survival on the market. Until 1983, hospitals in Germany could not make up a loss because the costs of previous years were refunded in the new year by calculating the daily rate accordingly. Even until 2003, it was rather difficult to run into a loss because, in most cases, the daily rates of the departments were calculated accordingly. However, since the introduction of DRGs, hospitals have to decide on the service portfolio, i.e., what products they want to offer for certain customers with certain needs. This is a new challenge for hospitals, and the answer to these questions goes far beyond the one-year-perspective.
A service portfolio is a typical instrument of strategic management that has only become relevant for hospital managers in the last decades. Until 1993, hospitals could not specialize on certain services but had to provide every service in their catchment area, which was obligatory at the level of the hospital. Currently, hospitals can specialize as long as the needs of the populations are covered. In the example of Figure 9 , the portfolio covers three departments (ENT, orthopaedic surgery and paediatrics) and analyses the marginal contribution and the number of competitors in the catchment area. The circles represent services, and the area of the circles is proportional to the turnover of this service.

Portfolio of a hospital. Source: own.
In this example, ENT has three different services. All of them have positive marginal contributions and should be sustained. Paediatrics has three services; two of them have a positive marginal contribution and one has a negative contribution. However, the latter is a unique service in the catchment area; i.e., it cannot be closed-down without bringing problems to the population. The other two services will have to subsidize this service. Orthopaedic surgeries also have a negative contribution, but none of them are unique in the catchment area. They can be closed without making patients suffer.
Portfolio analyses reduce complexity because norm strategies can be utilized for different constellations. Such a portfolio is highly relevant in zone III where short-term and deterministic solutions are not sufficient to cover the complexity and dynamics of the system. Instead, portfolios can be used as instruments of strategic management to make evidence-based decisions relative to the services provided.
4.2. Development of Innovative Implants
Therapy concepts with innovative implants are used more and more frequently in the treatment of chronic degenerative diseases, additionally reinforced by the high prevalence and further increasing incidence rate in the aging population [ 32 ]. In order to be able to meet these challenges adequately, a strategic approach in implant development management will be indispensable in the future.
From the initial idea of a physician or engineer of a new implant to the market-ready product and the implementation of the innovation as a standard therapy, there are many process steps to go through [ 33 ]. This includes phases of research and development, certification, reimbursement options and launch. The classic view of the implant development process ends with its adoption as a standard. However, improving the patient’s quality of life should play a decisive role in the development of innovative implants. Above all, the aim should be for the patient to use the implant for as long as possible after successful implantation. This adds a strategic dimension that expands the planning horizon by including the lifelong consequences of innovative implant.
For a long-term patient-centred perspective, specific aspects must be taken into account. First, the decision between doctor and patient of an implant must also be considered with regard to a benefit that may only occur later. Second, there should be an ethical assessment of the costs and benefits of current and future periods. Third, lifetime implants require more extensive clinical investigations and fatigue strength testing, which could create additional innovation barriers throughout the implant development process and need to be addressed.
In conclusion, a long-life perspective focused on the patient should be systematically integrated into the implant development process. This is based on several requirements for the implant, including durability, maintenance, interchangeability and compatibility with other implants and future therapies. In Figure 10 , an innovation model of the implant development process is shown, which embraced both strategic and operative management decisions. It enables a targeted orientation to the life perspective and an effective response to the high demands of an increasing residual lifetime after the first implantation.

Modified implant development process with long-life perspective. Source own.
Management in Dynaxity Zone III must take into account dynamics, complexity and uncertainty. As shown in the last section, this is already reflected in areas of healthcare. Another example is the high relevance of leadership in dealing with the COVID-19 pandemic. The greatly increased speed of interactions and the complexity of our societies require more strategic thinking when fighting pandemics than in previous centuries. Strategic COVID-19 management is not only a question of technical prognosis but also, in particular, is a question of communication, motivation and inspiration. The same applies to dealing with other “new” pathogens such as multi-resistant bacteria that healthcare facilities are confronted with today. A long-term strategy is required that takes into account the interactions between the various different health areas and the people actively addressed for networking.
5. Conclusions
It is obvious that the post-industrial society and economy are in Dynaxity zone III, which is characterised by high dynamics and complexity, which at the same time leads to an unknown degree of uncertainty without pausing stabilizing phases. Change is the “new normal”, and peaceful stability is the exception. The healthcare sector is no exception to this.
It must be understood that in today’s world is a system that cannot be fully described or analysed in a conventional manner as many new elements and dependencies are evolving and every action has an impact on many elements now and in the future. These challenges call for a response of the top management of nations, economies, health care services and all other institutions with a long-term perspective, consideration of interdependencies and synchronisation of different levels of plans. With the implementation of strategic management, the necessary long-term perspective is appropriately weighted and new analysis and planning tools are available. This has already been carried out in many areas of the health sector, as was demonstrated in this paper for several exemplifications. Other areas will inevitably follow.
At the same time, these new managerial and intellectual requirements pose a great challenge to our personal ability as human beings to design systems and organisations or to make meaningful decisions. The correct handling and use of information as well as the derivation of sustainable measures are prerequisites for strategic management, and employees are more indispensable than ever. Healthcare facilities such as hospitals must also be aware of this fact in their personnel policies and react to it. This includes investing in human capital by training and other educational opportunities to acquire comprehensive methodological and social skills. Ultimately, a completely new mindset and long-term and systematic thinking need to be established. What we require in health care—now more than ever—are co-workers with the ability to deal with complexity, survive under uncertainty, interrelate in networks and follow the values of health care with intrinsic motivation. Nobody has these strategic talents by nature, but we can foster, encourage and cultivate them in our collaborative cultures in the health care system.
Strategic management is based on strategic thinking. Consequently, any healthcare strategy must begin with a change in mindset or even the underlying paradigm. Strategic management is not primarily an application of management tools (although there is a lot to know and learn about these tools), but it is a mindset: the mindset for a dynamic, complex and stochastic postmodern world with ever-increasing speed, dependencies and uncertainty. These meta-parameters must come to mind for healthcare decision makers if they are to successfully manage change. Moreover, it helps to summarize these parameters in one concept or one word: Dynaxity. Therefore, knowing the fundamentals of Dynaxity can guide the thinking, decisions and actions of healthcare managers by directing their thoughts in the right direction.
Funding Statement
The project “Analyses of effectiveness and efficiency of regional MDRO-Networks—EARN” was funded by the BMG (grant: 2516FSB107). The ‘partnership of Innovation in Implant Technology (RESPONSE)’ was funded by the BMBF (grant 03ZZ0914C and 03ZZ0934A).
Author Contributions
Both authors have substantially contributed to the conception, writing and revising of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Language selection
- Français fr
Research powering innovation for Canada - NRC 2024–2029 Strategic Plan
Alternate format: Research powering innovation for Canada - NRC 2024–2029 Strategic Plan (PDF, 3 MB)
On this page
Executive summary, message from the president, introduction, strategic priorities, climate change and sustainability, health and biomanufacturing, digital and quantum technologies, foundational research, committing to excellence, achieving our vision.
This strategic plan for 2024–2029 will guide the National Research Council of Canada (NRC) as we advance and support important research and innovation. It outlines our commitments to Canada, our partners and ourselves and focuses on significant challenges facing the country. These challenges include climate change, strengthening Canada's life sciences capacity and bolstering the competitiveness of Canadian businesses in an increasingly uncertain and complex global economy. These challenges also represent opportunities for Canada, where new knowledge, technologies and innovations secure a prosperous future for our country.
As we work toward this vision of innovation and prosperity, partnership will play an important role. Canadian innovators, industry and government must come together and use the full range of our capabilities to capitalize on opportunities stemming from the accelerated pace of scientific discovery and technological change.
Our strategic plan presents a collective vision for the NRC. It reflects a year's worth of engagement across our organization and with our stakeholders about where there are opportunities for the NRC to have impact, how we can help position Canadian industry for growth and where we are positioned to help address key challenges facing our country.
Research and innovation priorities
Our strategic plan for 2024–2029 focuses on 4 intersecting research and innovation priorities with specific goals:
Canada is taking significant steps to address climate change and increase climate resilience. In doing so, there is also an opportunity to leverage the NRC's strengths to support a prosperous green economy. We can build upon Canada's established manufacturing leadership and further the development and adoption of technologies for vibrant advanced manufacturing industries. Industry and innovators need support to develop the new knowledge and technologies required to mitigate and adapt to climate change and to seize economic opportunities. The NRC is well positioned to provide this support. We will focus our capabilities and partnerships on decarbonizing 2 of Canada's key industries—transportation and construction, which represent a combined 8.7% of the country's gross domestic product (GDP)—and help adapt Canada's buildings, infrastructure and communities to climate change. Through this work, we will also support industries in taking leading technologies to market to compete on a global stage.
Canada's life sciences sector continues to grow, fuelled by leading talent. The COVID‑19 pandemic highlighted weaknesses and gaps in this sector but also opportunities to reinvigorate and rebuild Canada's capacity in this area. Investment in life sciences research and development is increasing and the sector's contribution to Canada's GDP has steadily increased by 25% over the last 5 years. Footnote 1 A strong and resilient life science sector depends on discoveries continually being taken from the lab to the market and made available to improve the health of Canadians. We will contribute our expertise and unique infrastructure to increase Canada's biomanufacturing capacity and focus on de‑risking and accelerating clinical adoption and commercialization of therapies and technologies that are user‑centric and affordable to produce at scale. Our ambition is to grow connected biologics, biomanufacturing and distributed care industries in Canada that are competitive and that are ready to address emerging health priorities and build our industry for the future.
Canada is a global leader in quantum and digital research, particularly in quantum computing, AI and machine learning. We are at a global tipping point with AI. And the coming intersection of AI, quantum and semiconductor technology creates significant opportunities for the NRC to position Canada to capitalize on the commercialization of this space. This is a growing and evolving sector, with quantum alone expected to grow to industrial scale in Canada by 2045. The NRC will focus on developing and advancing quantum and digital research and innovations toward market readiness and adoption. Through this, we will enable more productive and competitive industries and continue Canada's leadership in quantum and digital research excellence.
The Canadian research community, including the NRC's own researchers, needs specialized facilities and equipment to carry out scientific exploration. The NRC is the steward of Canada's participation in ground‑based observatories that are critical to the astronomy community. The NRC also maintains Canada's national measurement standards, which are essential not only for scientific research but also for many activities that affect the daily lives of Canadians, such as the dosing of medicines, ensuring fair trade and setting the foundation for standards and interoperability of equipment. Through this stewardship, we will continue to provide access to the world-class facilities needed to maintain Canada's reputation for scientific excellence, support emerging technologies and innovations and enhance the socio-economic well-being of Canadians. In doing so, we will continue to contribute to breakthrough scientific discoveries and innovative solutions.
Organizational priorities
Our research goals for the next 5 years are underpinned by organizational values and priorities that define who we are and that are foundational to our success. These organizational priorities help us continuously improve as a partner and an employer and drive us to make greater contributions to an innovative and prosperous Canada. These priorities are:
Health and safety
Our highest priority is the safety of our people and protecting our neighbours and environment. We will stay vigilant and nurture a culture of safety, security and protection that is part of the fabric of the NRC.
Support to business innovation
We support Canadian businesses so they can innovate and prosper. We do so by conducting research and generating valuable knowledge, funding research, providing access to research and small‑scale manufacturing facilities and connecting innovators in Canada and abroad. Through the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP), we provide critical funding and advice to help Canadian firms innovate and grow. We will continue to harness the full breadth of our activities and capabilities to help support business innovation and to rise to the challenge of increasing our economic impact.
Inclusive innovation
We aim to foster a diverse and inclusive workforce and workplace. We will do so while continuing to learn how best to use inclusive approaches to design our programs and services, guided by careful consideration of the different ways our work can potentially affect different groups. As part of this effort, we are committed to working towards excellence through partnership with Indigenous researchers and communities, and their Indigenous knowledge and knowledge systems.
Research excellence
Our commitment to and demonstration of research excellence is foundational to working at the leading edge of science and technology and to focusing this advantage on Canada's priorities. Research excellence makes us a sought‑after partner, assures our partners we will be a dependable part of their success and lets government know our advice is based on sound science. A culture of research excellence also enriches and sustains our organization by attracting and retaining world‑leading research expertise, helping create the NRC research leaders of tomorrow.
Organizational excellence
Our scientific activities, researchers and collaborations are supported by a comprehensive structure of teams and processes. These enabling functions range from procurement to program development and from communications and contracting to IT enablement and support. Within each area, we strive for excellence, as critical prerequisites for our success. Over the next 5 years, enabling functions will be key to the implementation of our process of scientific infrastructure renewal and to investments in IT. These investments will promote a secure cyber environment and allow us to incorporate intelligent digital tools into both our internal operations and our facilities renewal. The resulting enhancements will accelerate the speed of research and innovation.
This plan marks an important time for our organization as we drive Canadian research and innovation to address Canada's challenges and seize opportunities to secure a sustainable, inclusive, and prosperous future. During the pandemic, we rallied our people, assets and partners to answer the call of what were unprecedented challenges for Canada. This reinforced the power of the NRC as a national platform, with the people and relationships to orchestrate research capabilities, investments, and unique facilities and equipment to make a singular contribution to addressing Canada's priorities.
Although we have moved past the emergency of COVID‑19, there is work to be done to ensure a safe and prosperous country for generations to come. How will we strengthen our life sciences sector to deliver benefits for Canadians? What will we do to adapt to and mitigate climate change and to transition our advanced manufacturing industries to new low- and zero‑carbon products and services? How will we leverage the power of quantum and digital technologies to create more competitive, leading‑edge Canadian industries? The NRC will contribute to solutions to these challenges. Through our work, we will continue to conduct and support research and innovation that contributes to a more prosperous and more resilient Canada.
The NRC's role in supporting business innovation needs to take on renewed importance. With our unique and longstanding role in the innovation ecosystem, we will leverage our activities and capabilities to support businesses as they innovate, grow their ambitions and bring new products and services to market. The NRC Industrial Research Assistance Program (NRC IRAP) has been a hallmark of business innovation for more than 70 years. It is recognized as Canada's leading innovation assistance program for small and medium‑sized businesses, helping innovative firms succeed and grow. NRC IRAP will continue to support the most innovative Canadian businesses advance and commercialize new technologies. When this translates into increased demand for experts and advanced facilities to work on and test research and new technologies, the NRC will rely on its research and technical capabilities to answer the call.
Through our commitment to business innovation, we will support the success and growth of Canadian businesses that aim to be industry leaders, drive innovation and make an impact on domestic and global markets. These outcomes will help Canada's global position, grow our economy and improve the lives of Canadians.
Our capacity to support research and innovation will also increase through modernized facilities. Across the NRC, our people are energized to seize the historic opportunity of the government's 8‑year commitment of $962 million to recapitalize and renew our research facilities and infrastructure. This investment provides us with an opportunity to better support our clients and partners and to increase our capacity for collaborative research. Projects will align with our vision for the future, prioritize co‑investments with our partners and increase our capabilities by integrating digital tools.
Through the strategic planning process, we took stock of the world around us. We considered our strengths and those of our partners to determine where to prioritize our contribution to a prosperous, innovative Canada over the next 5 years and beyond. During a year of significant engagement, we heard from people throughout our organization and from key stakeholders across the Canadian innovation ecosystem. All of their voices have contributed to our vision for the next 5 years.
Our strategic plan is focused on challenges and opportunities in the priority areas of climate change and sustainability, health and biomanufacturing, and quantum and digital technologies. At the same time, our plan ensures we deliver research excellence in important foundational research areas.
It is my privilege to lead the NRC onward and drive execution on this plan. Our ambition is built on a foundation of leadership in research and innovation built over 100 years.
We are committed to meet the challenges of this moment, and deliver research that powers innovation for a more prosperous and sustainable future.
Mitch Davies President, National Research Council of Canada
Climate change, health and digital technology are areas that present both challenges and opportunities of great importance to Canada's future prosperity. To support the country's rapidly evolving innovation sector in addressing these challenges and seizing these opportunities, the National Research Council of Canada (NRC) must work more closely with its industry, academic and government partners than ever before.
This 2024–2029 strategic plan will guide us as we advance and support research and innovation that is important for Canada. It outlines our commitments to Canada, our partners and ourselves. It also reflects how the NRC will continue to evolve over the next 5 years.
Reflected in this plan is a collective vision developed through significant engagement across the NRC and with our stakeholders about Canada's key challenges and opportunities—and how to approach them.
This plan is grounded in our track record of important research breakthroughs and transformative technology development, made possible by the expertise and commitment of our people. It also reflects our long history of commitment to, and connection with Canada's entrepreneurs and innovators across multiple industries.
The NRC has supported research and innovation in Canada for more than a century and has taken on a variety of roles along the way to respond to the needs of the day. We are:
- an advisor and supporter of innovation by Canadian companies
- an ally to some of Canada's brightest minds in research in their pursuit of scientific excellence and breakthroughs
- a pioneer in research and development that has contributed to improving, changing and saving lives across Canada and around the world
- a steward of important and unique scientific facilities and equipment that help Canada align science and innovation with important priorities and challenges
Some of Canada’s most significant innovation breakthroughs came about in collaboration with the NRC, from the pacemaker and the electric wheelchair to childhood vaccines and attosecond lasers.
Our value as a resilient, reliable partner has been fundamental to our success in these roles. Uniquely positioned at the intersection of industry, academia and government, we contribute our capabilities to partners throughout the research and innovation ecosystem to achieve more for Canada together.
As we work to ensure Canada's long‑term prosperity, partnership will play an even greater role. We must come together and use all our strengths and resources to capitalize on the accelerated pace of scientific discovery and technological change. At the same time, there are multifaceted challenges on the road ahead, including mitigating and adapting to the effects of climate change, strengthening Canada's life sciences capacity, and bolstering the competitiveness of Canadian businesses in an increasingly uncertain and complex global economy.
Together with our partners, we are ready to take on these challenges and work toward achieving a more innovative and prosperous Canada.
NRC at a glance
A better Canada and world through excellence in research and innovation.
To have an impact by advancing knowledge, applying leading-edge technologies and working with other innovators to find creative, relevant and sustainable solutions to Canada's current and future economic, social and environmental challenges.
Integrity • Excellence • Respect • Creativity
- 2,293 scientists, engineers and technicians
- 269 NRC IRAP ITAs
- 87 nationalities in our workforce
- 39.5% women in our workforce (relative to Canadian market availability: 38.2%)
- 542 students, postdoctoral fellowships and research associates (hires)
- 24 laboratory sites
- 106 NRC IRAP points of service
Scientific achievements
- 1,222 peer-reviewed publications
- 1.19 citation score relative to world average
- 10.9% with United Kingdom
- 8.5% with Germany
- 5.2% with Japan
In 2022-23, the NRC:
- filed 267 patent applications
- 606 active patents currently licensed
- 461 patent families
R&D clients and collaborators
- 89% clients say the NRC helped them achieve results Footnote 2
- 1,005 R&D projects for clients ( 969 R&D clients)
- 379 active collaborative R&D projects ( 116 funded collaborators)
Industrial Research Assistance Program (NRC IRAP)
- 3,486 funded firms
- 6,204 firms received advisory services only
- 13,973 total jobs supported
- 35% total revenue growth of client firms
- 21% employee growth of client firms
The NRC is involved in a wide range of research and innovation activities across a broad set of areas of expertise and application. Over the next 5 years, we will focus on areas that require immediate attention and where our collective efforts can have the greatest impact.
Our plan for 2024–2029 focuses on 4 intersecting research and innovation priorities:
- climate change and sustainability
- health and biomanufacturing
- digital and quantum technologies
- foundational research
These areas represent the intersection of government priorities, industry needs and areas of NRC excellence. Multifaceted issues related to computing power, big data, connectivity and telecommunications, artificial intelligence (AI) and smart systems, new materials, life science technologies, and the need for new forms of low‑carbon technologies all offer opportunities for innovation in the coming years and opportunities to secure Canada's future prosperity. With our partners, we can leverage our existing strengths and focus our capabilities to help address these challenges.
We recognize the dynamic nature of government priorities and the evolving nature of emerging technologies and industry needs. Therefore, while our plan will guide us over the next 5 years, it will also allow us to be agile. This will enable us to adapt and address needs and opportunities as they emerge.
This plan sets goals for each of our 4 research and innovation priorities, along with strategies that will help us increase our impact and the value we deliver to Canadians.
Our ambitions for the next 5 years go beyond innovation and economic outcomes. As an organization with more than 4,000 employees, it is equally important to foster the organizational priorities and values that are foundational to our identity as an innovator and preferred partner. To that end, this plan also focuses on strengthening our connection to industry as well as on health and safety, inclusive innovation, and research and organizational excellence. These organizational priorities will keep us on course, provide the support needed to strengthen Canada through research and innovation, and ensure we have a workforce with the capabilities and commitment to achieve our goals.
Strategic plan at a glance
Accelerate the decarbonization of Canada's transportation and construction industries
Help Canada's buildings, infrastructure and communities adapt to climate change
Enable the development and rapid manufacturing at scale of novel vaccines, therapeutics and other bio-products
Develop next generation precision tools/devices for distributed diagnosis and therapies and enable their clinical use and commercial adoption
Advance quantum science towards viable technologies for commercialization and application in priority areas for Canada
Lead digital research and innovation to facilitate high-quality solutions to critical challenges and jumpstart industry adoption
Effectively fulfill our roles in national astronomy assets and measurement
Protect our people, neighbours and environment
Strengthen our connection to industry for greater economic impact
Lead workplace diversity toward inclusive innovation
Commitment to world leading advances in technology, research and innovation.
Strive for excellence in our enabling teams and supporting business procedures
Climate change is affecting Canada's communities and industries in increasingly visible ways. Along with bringing the need to mitigate and adapt to these impacts, climate change also presents growing opportunities for Canada to diversify its economy and build on existing strengths to lead in emerging industries.
Industry has an integral role to play in Canada's climate response and in building a prosperous green economy. However, industry needs support to develop the new knowledge, technologies and innovations required to meet Canada's goals and to be leaders in their fields. Such developments will be crucial to reducing greenhouse gas emissions and protecting Canadians against the impacts of climate change in ways that are economically, environmentally and socially sustainable. The transition to a green economy also holds the promise of future gains for Canadian industries, including increased gross domestic product (GDP), more clean technology exports and up to 400,000 new jobs by 2030. Footnote 3
The NRC is well positioned to provide this support. In partnership with innovators and researchers from industry, academia and government, we will employ our research strengths, expertise and infrastructure in large multidisciplinary initiatives to help discover, develop, evaluate and deploy new technologies and innovations that can address Canada's climate change priorities and jumpstart the green economy. Particular focus will be on decarbonizing key Canadian industries, as well as on strengthening the climate resilience and adaptability of Canadian communities and crops. Through this work, the NRC will contribute to Canada's transition to a prosperous green economy and to improved climate resilience. We will also help Canadian industries take leading technologies to market and compete on a global stage.
Goal 1: Accelerate the decarbonization of Canada’s transportation and construction industries
Industrial decarbonization is critical to mitigating climate change. Canada has an opportunity to build on its strengths in manufacturing and support the transition of advanced manufacturing to low‑carbon technologies. Over the next 5 years, the NRC will focus on decarbonizing 2 key industries in Canada: transportation and construction. Together with our partners, we have the critical mass, expertise and facilities to make an impact.
Two of Canada’s key industries are large greenhouse gas emitters: Footnote 4
Aerospace and automotive
Share of Canada’s GDP: 1.3%
Share of total greenhouse gas emissions: 22%
Construction
Share of Canada’s GDP: 7.4%
Share of total greenhouse gas emissions from buildings: 13%
Canada's transportation and advanced manufacturing industries are traditionally strong but must seize opportunities to successfully transition to a low‑carbon future. Aligning with recent legislation introduced around the globe will require manufacturers to reduce their carbon output and to shift to zero‑emission vehicle production. To keep up with the global transition to a green economy and strengthen Canadian competitiveness, the industry will need technological solutions to overcome obstacles such as the deployment of charging and fuelling infrastructure, the supply of critical minerals for batteries and the need to develop cleaner fuels.
Construction is another traditional industry facing similar challenges in this rapid transition. Meeting Canada's 2050 net‑zero target will require a paradigm shift in how industry develops and adopts new construction technologies, practices and regulations. To seize opportunities and lead globally, Canada's construction sector must accelerate the testing, adoption and scaling of innovative new methods of homebuilding that are also climate resilient.
Our longstanding and new partnerships, strong industry connections and planned investments provide an opportunity to make a difference in enabling these transitions. We envision prosperous Canadian industries, with the NRC supporting the development and implementation of green technologies and solutions.
Supporting strategies
Create research and technical solutions that address barriers to the adoption of electric vehicles.
We will focus on mobility electrification, including batteries and powertrains, as well as the development, optimization and safe integration of low‑carbon technology and light-weighting, aerodynamics and sustainable fuel approaches. We will also leverage these efforts and technologies to advance lower‑emission aircraft and marine vessels.
Fill a critical gap in the battery materials supply chain
We will work on solutions to reduce the cost of materials needed for battery production, in turn reducing the cost of vehicles. We will work to improve mid‑stream processes and technologies to fill the gap between resource extraction and battery materials supply, and explore battery recycling as another avenue for increasing supply chain.
Support the development and use of low‑carbon construction materials and systems
We will provide the knowledge and data required to identify and develop low‑carbon materials, products, services and tools, and to support industrial development of low‑carbon and zero‑carbon construction materials. We will also improve decision support tools for economical and socially responsible low‑carbon building and infrastructure, from design through to end‑of‑life management.
Leverage digital technologies to drive innovation and productivity in low‑carbon construction
We will develop digital, fit‑for‑purpose solutions to empower construction professionals in the transition to low‑carbon operations. This includes working with industry and academia to reduce the time required for permitting and inspections through e-permitting and virtual inspections, reduce costs for construction through digitalization and advanced manufacturing (e.g., offsite, 3D printing), and reduce costs through decreased material waste.
These efforts will support more efficient and resilient construction.
Goal 2: Help Canada’s buildings, infrastructure and communities adapt to climate change
Canada's buildings and public infrastructure systems—including bridges, roads, and water and wastewater systems—are guided by codes and standards based on historical climate data. In many cases, these assets were not designed to withstand the extreme weather of today, let alone the even more severe weather anticipated in the future.
The impacts of climate change and extreme weather events are becoming more frequent and their effects on the daily lives of Canadians are intensifying. Understanding how to adapt and protect infrastructure and homes is key to developing and adopting innovations needed to create a resilient built environment.
Increasing the climate resilience and adaptability of Canada's food security is also a priority. Climate change is expected to adversely affect food production and prices, food distribution systems and certain aspects of food quality. Many crop yields are predicted to decline due to the combined effects of changes in rainfall, severe weather events, and increasing competition from weeds and pests on crop plants. Livestock and fish production are also projected to decline, with prices expected to rise in response to the decreased supply.
We strive to support a strong domestic food supply to nourish Canadians across the country and to help protect them from the elements in their homes, buildings and communities. Our work will also help create export opportunities for Canadian industries as other countries seek Canadian-made solutions to feed their communities and adapt to climate change.
Integrate climate resilience into the design of and standards for buildings and infrastructure
We will provide the knowledge needed to integrate climate resilience into design, guides, standards and codes for buildings, homes and infrastructure. This will help Canada's built environment better withstand future weather events and support the adaptation of Canadian homes to climate change.
Develop nature‑based solutions to protect coastal infrastructure
We will learn from nature and improve our understanding of the natural capabilities of beaches, wetlands, marshes, barrier islands, reefs and headlands to manage the risk of coastal flooding and erosion. These solutions are often more sustainable and adaptable to changing environments than conventional, human‑made protective structures.
Support technology and data‑informed approaches to climate‑resilient crop design
We will advance a platform of integrated technologies to help the indoor agriculture industry accelerate crop design and develop novel engineering technologies in support of regional food production. We will also use digital tools, including simulated environment modelling and data integration, to improve the resilience of Canadian crops to climate change.
Improve the competitiveness and sustainability of ocean‑derived products
We will advance technologies for sustainable climate adaptation and effective use of marine bioresources. Our focus will be on ocean health monitoring and modelling, the full use of all extracted and cultivated bioresources, and the development of high‑value marine bioproducts.
Canada's life sciences sector is growing, strengthened by leading talent. The COVID-19 pandemic highlighted weaknesses and gaps in the sector, such as Canada's overreliance on imports of critical health products and supplies. But it also provided opportunities to reinvigorate and rebuild the country's life sciences capacity. Research and development investment is increasing, and the sector's share of Canada's GDP has steadily increased by 25% over the last 5 years. Footnote 5 Ensuring the required expertise, resources and governance is important to maintain the sector's growth. This will also allow for a fast and effective response to the next health crisis as well as rapid advancement of cutting‑edge technologies for vaccines and treatments, which can then be quickly transferred to industry for efficient, scaled‑up production.
New therapies and technologies are also needed for modern and efficient health care and to build the life sciences industries of the future. Canada's health-care system is challenged with increasing demands and is heavily dependent on importing expensive medicines for many chronic and rare diseases. Reducing costs—and more importantly, increasing access to health care—requires innovative solutions and new technologies that are affordable, fit for purpose, manufactured domestically and deployed in a distributed care model across Canada's vast geography.
A strong and resilient sector depends on continuous discoveries taken from the lab to the market and made available to improve the health of Canadians. The NRC has a unique role in Canada's collective effort to achieve this vision. We will contribute our expertise and unique infrastructure to increase Canada's biomanufacturing capacity in preparation for the next health emergency. We will also focus on de‑risking and accelerating clinical adoption and commercialization of technologies that are user‑centric and affordable to produce at scale. Our ambition is to contribute to the thriving biologics, biomanufacturing and distributed care industries in Canada that are ready at scale to address emerging health priorities.
Goal 3: Enable the development and rapid manufacturing at scale of novel vaccines, therapeutics and other bio-products
Biomanufacturing goes beyond vaccines and therapeutics
Our atypical fermentation facility on Prince Edward Island specializes in the development and scale-up of fermentation processes to add value to renewable marine and agricultural bioresources. The work at this facility supports Canada’s food security by helping increase the country’s biomanufacturing capacity and contributing to advances in marine and agricultural bioresources.
Canadian researchers are responsible for many important breakthroughs in vaccines and therapeutics, from the discovery of insulin over a century ago to the development of vaccines for polio, meningitis and Ebola. While there continues to be a strong community of academic and industry innovators focused on the life sciences here in Canada, the country has lost much of its domestic capacity to produce commercial medicines at scale. Canada's consumption of vaccines and therapeutics increased substantially in just over 20 years, from $473 million in 1997 to $4.8 billion in 2019. However, Canada imports 85% of the vaccines and therapeutics it needs today, up from the 19% imported in 1973. Footnote 6
The COVID‑19 pandemic revealed Canada's biomanufacturing weaknesses at the same time as it highlighted the critical role of vaccines in effective pandemic response. Canada's approach has been to leverage its existing strengths and expertise to rebuild capacity in the domestic biomanufacturing and life sciences sector. Canada's Biomanufacturing and Life Sciences Strategy provides a blueprint to revitalize the sector and better prepare for future health emergencies by investing in research systems and the talent pipeline, growing businesses, building public sector capacity and enabling best‑in‑class clinical trial infrastructure.
This strategy identifies the NRC as a cornerstone of intramural research and development, providing expertise, infrastructure and other important capabilities to enable Canada's approach to creating a strong domestic biomanufacturing ecosystem. We will use this long‑standing expertise and value proposition to help Canadian industry advance a robust pipeline of biologics and foster at‑scale manufacturing and commercialization of those innovations in Canada.
Support accelerated development and production of Canadian made vaccines and biologic medicines
We will accelerate the development of Canadian made biologics, including vaccines and innovative therapies. Our new Clinical Trial Material Facility, the only public facility of its kind, will serve as a product development bridge for domestic production of vaccines compliant with good manufacturing practice (GMP) regulations and other biologics material for first in human clinical trials. This expertise can then be transferred to other contract manufacturing organizations—such as the new Biologics Manufacturing Centre (BMC), funded as part of the Government of Canada’s investment in biomanufacturing—to produce materials for commercial products.
Contribute our capacity and capabilities to meet pandemic preparedness ecosystem needs
We will re-invest in our research and development infrastructure and leverage our research expertise and training capacity to forge new partnerships with Canada Biomedical Research Fund hubs across the country, and to enable rapid advancement of platform technologies and their high-risk projects. The collaborations will de risk and accelerate the development of vaccines and biologics and promote their transfer to industry to address current and future health emergencies, including antimicrobial resistance.
Goal 4: Develop next generation precision tools and devices for distributed diagnosis and therapies, and enable their clinical use and commercial adoption
Health care is the top concern of Canadians across the country, Footnote 7 with cost pressures and chronic staff shortages leading to strained resources as well as declining and inconsistent quality of care. At $344 billion in 2023, health‑care spending represents a significant share (12%) of the country's GDP. Much of this spending is related to hospital and medication. Footnote 8
Reducing health‑care system costs and increasing access to health care demands innovative solutions and the development of new, easy‑to‑use technologies. One innovative approach is distributed care, which improves accessibility by promoting a network of decentralized care services located closer to the patients in need. Increasing cost pressures, combined with the public health restrictions put in place to slow the transmission of COVID‑19, have already pushed health‑care systems toward distributed care. The ongoing success of this model depends on mass production and use of efficient, cost‑effective precision tools and devices to monitor, assess and diagnose patients.
With our industry and clinical partners, we will focus on the development, clinical adoption and commercialization of precise and user‑centric diagnostic technologies, facilitate accessibility at scale for diagnosis and assessment, and create new tools for patient management and therapy pathway optimization. We are aiming for a future where all Canadians—rural and urban, across all regions—have ready access to leading‑edge diagnostics and therapies to improve their quality of life.
Support the development and adoption of innovative point-of-care diagnostics
To enable clinical adoption and at-scale industrial commercialization, we will invest in biofabrication capacity for microfluidic devices and also build on our strong collaborations for advancing molecular diagnostics and organ-on-a-chip. Our new cleanroom infrastructure will host a pilot-level capacity for the fabrication, assembly and quality control of microfluidic devices.
Increase the affordability and accessibility of innovative therapies
We will develop and advance disruptive platform technologies for biological cell and gene therapies to accelerate discovery, development, manufacturing and translation to clinical trials of innovative precision medicine. We will coordinate a national effort to increase the affordability and accessibility of these technologies, in collaboration with academic facilities, networks, clinicians, hospital centres and other partners.
Develop user-centric digital health and virtual care technologies
We will increase our efforts for clinical adoption of our digital health and virtual care technologies to meet the growing need of an aging population to access user-centric hybrid care that combines virtual and in person care. This includes technologies that enable remote physiological monitoring of patients to assess their health status noninvasively, which will help improve aging in place and accessibility to timely clinical assessment.
Digital and quantum technologies are changing the world at an accelerating rate. Countries around the world are investing to develop technologies to improve economic performance, increase competitiveness, and enhance the well‑being and security of their citizens. The cross‑cutting nature of these technologies means they will ultimately affect all economic sectors and bring significant advances to many fields.
Canada is currently a global leader in quantum and digital research, particularly in quantum computing, artificial intelligence and machine learning. We have the opportunity to translate these discoveries into commercially viable technologies and help Canadian industries adopt technologies that improve their productivity and competitiveness.
The NRC has the expertise, facilities and partnerships required to develop and advance quantum and digital research, and to advance promising innovations toward market readiness and adoption. This continuum of capabilities will enable more productive and competitive Canadian industries through the commercialization and adoption of digital and quantum technologies. It will also enable Canada's continued leadership in quantum and digital research excellence.
Goal 5: Advance quantum science toward viable technologies for commercialization and application in priority areas for Canada
The quantum sector is growing quickly and is expected to reach industrial scale in Canada by 2045. Quantum applications and innovations are also expected to drive advances in areas of critical importance for Canada and beyond, including health care, climate change, transportation and cybersecurity.
The NRC will focus on developing and advancing quantum and digital research and innovations toward market readiness and adoption. Through this, we will enable more productive and competitive industries and continue Canada's leadership in quantum and digital research excellence.
Historically, Canada has been a leader in quantum science. Our country is home to a growing quantum ecosystem with university‑based centres across the country and an increasing number of quantum‑focused companies. Between 2016 and 2019, there was a 58% increase in quantum firms and a 59% jump in quantum‑related jobs. Footnote 9 Canadian companies such as D‑Wave, 1QBit, Xanadu and Photonic are now recognized as global leaders in quantum computing and software. Canada also boasts healthy private and public sector investments. That includes more than $1 billion from the federal government between 2012 and 2021, more than $1 billion since 2002 from private investors and provincial investments in centres of quantum leadership across the country.
As other countries ramp up their investment and focus on quantum science and technology, Canada must keep pace to maintain its leadership position. Through the $360 million National Quantum Strategy (NQS), Footnote 10 Canada is building on and leveraging its strengths to translate scientific leadership into innovative, market‑ready technologies with the potential for strong economic benefits.
We will orient our quantum science and technology activities to support the implementation of the NQS and its focus on sensing, computing and communications. In doing so, we will support Canada in realizing the economic potential of quantum technologies. We will also deploy our scientific knowledge, infrastructure, talent and deep connections in the quantum ecosystem (domestically and internationally) to advance technologies along the path to commercialization.
Develop next-generation quantum sensors for health care, defence, transportation and environmental sensing applications
We will help develop a new generation of sensor systems that perform beyond the limits of classical physics and may be engineered and commercialized for priority application areas that benefit Canadians. This includes advancing photonic based quantum sensing technologies and the transition of quantum systems onto chips.
Apply commercial quantum computing solutions to create ground-breaking innovations for key economic sectors
We will help maintain Canada’s global leadership in quantum research and the commercialization of quantum computing applications by capitalizing on the recent progress made by intermediate scale quantum computers available through cloud platforms. This includes developing and applying new quantum algorithms, simulations and software that harness the power of these new computing capabilities.
Build revolutionary quantum capabilities by developing interconnected quantum devices communicating at a distance
We will work to scale quantum technologies from components into interconnected quantum systems with the overarching goal of enabling secure quantum communication at a distance. These efforts will form the building blocks of more powerful quantum systems by getting heterogeneous devices to speak to each other while preserving the exchange of quantum information. Testbeds will be used to de risk the interoperability of quantum components and inform standards.
Goal 6: Lead digital research and innovation to facilitate high-quality solutions to critical challenges and jumpstart industry adoption
Digital technologies are critical to maintaining Canada's competitiveness and productivity across a range of sectors. An estimated 70% of new business value over the next decade will come from digitally enabled platforms, and 30% of global corporate revenue will come from digital ecosystems by 2025. Footnote 11 These technologies are also transforming the speed and process of research, with faster use of data, faster computers and multidisciplinary teams leading to more rapid discoveries and technology developments. Digital technologies can also be applied to accelerate the adoption and implementation of innovations and solutions in critical areas such as climate change and health care. By 2024, it is expected that 30% of new drugs and materials will be systematically discovered using generative AI techniques. Footnote 12
While already a leader in digital research, Canada needs to accelerate commercialization and adoption of these technologies and innovations at scale. For instance, the aerospace industries in Germany, Japan and the United States are shifting toward aircraft certification through virtual testing and digital twinning, which is significantly more cost- and time‑efficient than physical testing. Canadian industries need support to realize the transformative benefits of the digital applications and technologies available to them, and how to incorporate them into their operations.
Work toward our digital transformation
Developed in alignment with this strategic plan, our digital transformation vision will enable a digital research environment in which data are mobilized for high quality, innovative research and solutions. Activities will also contribute to the achievement of strategic goals related to industrial decarbonization, health and biomanufacturing. The following activities include some of the key elements of our vision for digital transformation.
While technological advances have created opportunities for increased innovation and economic growth, they also bring public policy challenges. Policies and regulations around generative AI technology must be designed to foster economic growth in the AI field while also protecting government, companies and Canadians. With its successful history in AI technology development, Canada has the opportunity to take on a leadership role with its partners and allies and develop AI tools that are productive, fair and secure.
The NRC has more than 30 years' experience in developing and deploying digital technologies that could be adapted to improve the advanced manufacturing, life sciences and transportation sectors, including the digital twins that will enhance the NRC's aerospace testing capacities. These technologies can also be used to support other national priorities. For example, our capabilities in the preservation of Indigenous languages using AI are helping to strengthen Canada's relationships with Indigenous peoples. In all these technologies, we will develop safe and responsible AI‑powered solutions that align with Canadian values and those of the NRC.
Leveraging our expertise, we will work with partners to develop high‑quality and fit‑for‑purpose solutions, support and de‑risk the continuing digital transformation of industry and government, and develop and commercialize technologies that collect and use data in a way that preserves both privacy and trust.
Integrate more digital capabilities into our facilities
We will make strategic investments in digitalization as we systematically renew our facilities. This will boost our ability to accelerate scientific discovery for the development and availability of trusted solutions as well as our ability to demonstrate to industry the transformative potential of digital technologies and applications. Specifically, digital capabilities such as sensors, data installations, and advanced computer capacity for modelling and automation will add new functionalities and possibilities to our research and development activities.
Develop trustworthy, privacy preserving and secure AI powered technologies
As we continue to develop digital technologies and capabilities, we will focus on research that ensures the safety and trustworthiness of AI powered solutions. This includes research and development of encryption methods that are resilient to quantum computing threats, privacy enhancing technologies, methods to better manage security vulnerabilities, and approaches to making robust AI systems that are safer and more transparent.
Provide services to industry and government that support digital solution adoption
We will invest in current and expanded activities and services that provide the guidance, assessments and solutions required to facilitate digital transformation by government and industry. We will lend our technical and advisory expertise for the adoption and scaling up of unique, fit for purpose solutions to improve their operations or products. In addition to providing digital assessments, we will also develop and provide services that focus on cybersecurity, privacy protection and responsible AI.
The facilities and equipment needed by our own researchers and the broader Canadian research community are key to enabling scientific exploration that leads to breakthroughs. Some infrastructure is of such scale that it requires global cooperation to develop and maintain. The NRC plays an important role in these endeavours.
We represent Canada’s participation in ground-based observatories that are critical to the astronomy community. The NRC also maintains the national measurement standards that are essential to both scientific research and daily life in Canada, including the dosing of medicines, ensuring fair trade, and setting the foundation for standards. Through this stewardship, we provide access to the world-class facilities required to maintain Canada’s reputation for scientific excellence, support emerging technologies and innovation, and enhance the socio-economic well-being of Canadians. In these roles, we have contributed to breakthrough scientific discoveries and innovative solutions to global challenges and accelerated the adoption of emerging technologies.
Goal 7: Effectively fulfill our roles in national astronomy assets and measurement standards to maintain Canadian scientific and technology leadership
Astronomy is one example of how we support foundational science, both through the research we conduct and through the facilities we manage and operate for use by the scientific community. Canada is a recognized world-leader in astronomy research and technology and home to unique research, development, innovation and testing capabilities as well as incredible talent. Over the past decades, Canadian astronomers have made many important technological breakthroughs like advanced optics, composite radio reflectors and digital signal processing systems. They have also made many important discoveries, from fast radio bursts to exoplanets.
Membership in the Square Kilometre Array Observatory (SKAO)
The NRC’s membership in this global radio astronomy observatory with 16 partner countries will enable transformational science about the universe, exploring questions such as how and when the first stars and galaxies were formed and how planets form. As a member, Canada will establish an SKAO regional data centre, and the NRC will provide in-kind contributions for the telescope.
To continue this pursuit of excellence, researchers need access to a range of world‑class facilities in strategic locations across the globe. Under the National Research Council Act , we are mandated to operate and administer Canada's national observatories. As part of this role, we also manage Canada's participation in international ground‑based facilities and provide merit‑based access to these observatories for Canada's astronomy research community.
Metrology is another example of our foundational research, beginning with the determination of measurement standards through to the application of those standards. Precise measurements are essential for medicine, trade, technology and engineering—areas that contribute to health, prosperity, quality of life and environmental protection.
The NRC and Measurement Canada form Canada's national measurement system. We act as the country's national measurement institute and under the National Research Council Act , we steward Canada's practical standard realizations of the International System of Units (SI). In this capacity, we work with the national measurement institutes of other countries to redefine the base system of measurements. We also work with other organizations around the world to provide metrology research and services that help transform ideas into market‑ready technologies of benefit to society, the economy and the environment.
Provide Canadian researchers with access to world-class astronomical observatories and expert support
We will provide on-site observatory staff and distributed operations for Canadian observatories, including maintaining a unique site for community radio telescopes. We will also provide large-scale scientific computing infrastructure as well as specialized astronomy data management expertise and support for instrumentation development projects. In addition, we will help maintain Canadian excellence in astronomy and physics by enabling the meaningful participation of Canadian researchers in international observatories and collaborations.
Design, build and deploy new and innovative astronomical instruments
We will work with academic and industry partners on cutting-edge designs that enable the community to perform research. We will look to apply the findings from successful collaborations with industry in other sectors to advance astronomy and foster business innovation. At the same time, we will work with industrial partners to adapt technologies initially developed to advance research in astronomy and astrophysics for use in other industries.
Advance leading-edge measurement science to define the International System of Units
We will conduct strategic research to advance unit realizations, dissemination methods and measurement solutions, aiming to push the boundaries for international metrology. This includes playing a leading role in the approved international roadmap for the redefinition of the SI second by advancing our research on portable optical clocks. To advance the digital transformation of metrological infrastructure, we will also establish a machine understandable digital framework for data representation.
Develop new measurement standards to accelerate the adoption of emerging technologies
We will develop new measurement standards and instruments to provide the foundation needed to support Canadian priorities. These include measurements and standards for next-generation power electronic modules and rapid charging stations for electric vehicles as well as standards for emerging quantum technologies.
The NRC's mission is to advance science and technologies to find innovative solutions for Canada. How we approach our work and the values that underpin our everyday activities are equally important. These organizational priorities and values give our work focus, help us continuously improve as a partner and as an employer, and drive us to make greater contributions to an innovative and prosperous Canada.
Over the next 5 years, we will continue to emphasize the key organizational priorities that have made us who we are and remain foundational to our success.
Protecting our people, neighbours and environment
We are involved in a variety of research areas, each of which present their own set of health and safety considerations. These range from employee health and protection to animal safety, from radiation safety to hazardous materials management. As an organization, we need to stay vigilant and continuously work to meet the highest standards for health and safety.
We strive to maintain a safe work environment, but we know we need to continuously improve. Our efforts over the next 5 years will be aimed at fostering a culture of safety and vigilance that is woven into the fabric of the NRC. Our objectives are threefold:
- we will provide all NRC employees with the training and tools needed to apply the highest level of safety in their work.
- we will be a model for safe workplaces.
- we will be a leader in environmental practices.
During the past year, we assessed the current state of our health and safety culture and identified opportunities for improvement. We will carry on with this work by keeping communication lines open, sharing knowledge and focusing on learning what we need to do better. These efforts will include creating targeted information and awareness campaigns, training, monitoring, and applying effective oversight. In the communities in which we operate, we will strive to be an even better neighbour by continuing to assess, monitor and manage risks, and by remediating contaminated NRC sites to reduce potential environmental and human health risks.
Our intention is to deepen our culture of excellence for health, safety and environmental stewardship, and to place health, safety and environmental considerations at the forefront of planning for all new activities at the NRC.
Strengthening our connection to industry for greater economic impact
Supporting Canadian businesses in their efforts to innovate and prosper is central to what we do. Examples of these efforts include:
- conducting research, generating valuable knowledge, and securing new Canadian intellectual property that leads to new products and services for Canadian companies
- providing access to small‑scale manufacturing and other facilities to develop, prototype and scale up new technology
- connecting Canadian innovators to leading research and development expertise and facilities
- enabling cross‑sector partnerships and collaborations in Canada and abroad
- providing vital funding and advice to advance innovative research and development projects
The 2022 federal budget recognized the NRC's importance to Canada's innovation ecosystem and challenged us to increase our economic impact. This challenge has been central to our strategic planning process, including how we can improve our engagement with Canadian industry and better support companies in the commercialization of their technologies and innovations.
The NRC IRAP has played a considerable role in the success of Canadian business innovation over the past 75 years. Touching close to 10,000 firms per year through funding, advice and connections, NRC IRAP support has resulted in significant success for small and medium‑sized enterprises both domestically and internationally. NRC IRAP will play an integral role in our approach to supporting business innovation.
Elements of the NRC's approach to increasing engagement and economic impacts include:
- using the full breadth of our capacity, expertise and connections to support and stimulate Canadian business innovation
- further leveraging our Challenge programs as collaborative platforms to strengthen our ties with universities, colleges and Canadian companies, and to advance projects with vast market potential
- being the partner of choice for Canadian small and medium‑sized enterprises by increasing access to our assets and providing them with the specialized expertise, scale and resources they need to take technologies to market
- focusing on leveraging the untapped potential of our proprietary technologies and patents, further increasing the impact of our research achievements for Canadians
Strengthening connections with our international partners for greater collaboration, export opportunities and to position Canada as a global player is also an integral part of our business engagement plan. We will focus on priority countries and identify greater opportunities to collaborate in strategic areas, including electric vehicles, climate change and semiconductors.
Through these efforts, we aspire to work with the most innovative firms in Canada, be a connector for knowledge translation and technology adoption, and facilitate the commercialization of technologies that will position Canada as a leader in innovation.
Leading workplace diversity toward inclusive innovation
We recognize the importance and benefits of working in a diverse and inclusive environment. Cultivating cultural understanding brings acceptance, empathy and growth. As a research and innovation organization, the NRC understands that diversity also enhances the quality of our work. It has been proven time and time again that diversity unlocks innovation—and that the most innovative enterprises tend to be the most diverse.
We are only as good as our people, so we are committed to the growth and development of everyone working across our organization. By upholding this commitment, we will make the NRC a workplace of choice recognized for its inclusive culture of growth and excellence. The NRC will be a place where people can pursue passions, explore possibilities and develop the skills that are indispensable to our partners and critical to advancing excellence.
Over the past few years, we have made important progress on building an even more diverse and inclusive workforce. We have reached or exceeded a level of representation for women and racialized persons that reflects labour market availability, and we will continue to work to attract and retain more diverse people, including persons with disabilities and Indigenous persons.
Inclusive innovation reinforces our organizational values
Integrity: Behaving ethically, honestly and objectively at all times; being impartial and transparent with colleagues, collaborators, stakeholders, clients and the people of Canada; and exercising sound stewardship of our resources.
Excellence: Pursuing excellence in all that we do—in our research and innovation, in our collaborations, in the execution of our programs, in our support to firms and in the delivery of our common corporate services.
Respect: Valuing and respecting the knowledge, expertise and diversity of our colleagues, collaborators, stakeholders and clients, and their ability to have an impact on Canada and the world.
Creativity: Harnessing our imagination and our passion for scientific excellence, exploration and innovation to generate new knowledge, new technologies, new business processes and new collaborations for a better NRC and a better world.
But building a diverse workforce is only the first step. Over the next 5 years and beyond, we will pursue inclusive innovation. This approach moves beyond the internal focus of creating an inclusive workforce and workplace to also focus externally on how we conduct our work and our impact on diverse communities.
Sustainable change can happen only through a collective, ongoing commitment across the NRC. This includes supporting all employees and leaders to learn, reflect and adapt, ensuring we all contribute to an inclusive, barrier‑free workplace where everyone feels welcome, respected and valued. We will also learn to use inclusive design approaches to conceptualize and develop our interdisciplinary programs and services, carefully considering the different ways our work can potentially affect different groups.
Indigenous engagement is a significant aspect of our commitment to inclusive innovation. Over the next 5 years, we will work to increase both our capabilities and our opportunities to undertake meaningful engagement with Indigenous peoples and communities. This will help inform our business and research practices, and support the representation of Indigenous peoples in our workforce and in the broader research and innovation system. As the NRC operates sites across Canada, we acknowledge the diversity of Indigenous territories and communities where our research and innovation activities are conducted. Accordingly, our commitment to Indigenous‑inclusive innovation is responsive to the distinctiveness of Indigenous peoples with whom we engage all across Canada.
This past year, the NRC has engaged experts and resources to help guide us on the journey to making this vision a reality. Some of our commitments on this journey will include:
- building intentional relationships and partnerships with Indigenous researchers, innovators, communities, organizations, businesses and governments
- advancing equity and reconciliation through collaboration and partnerships centred on Indigenous priorities
- bridging knowledge systems to enhance existing research and operations
Progress in these areas will bring us closer to being inclusive and representative of Indigenous peoples and help us achieve excellence through partnerships with Indigenous researchers and communities.
Research excellence refers to our commitment to achieving world‑leading advances in technology, research and innovation. It is a commitment to achieving high rankings on comparative measures of impact, using both established and progressive assessments. We strive for excellence in all that we do to create outcomes of the highest quality that lead to impact.
Our commitment to and demonstration of research excellence assure our partners that we can be a critical, dependable part of their success and lets government know that our advice is based on sound science. A culture of research excellence also enriches and sustains our organization by attracting and retaining world‑leading research expertise, helping to create the NRC research leaders of tomorrow. Collaborators worldwide will increasingly seek to work with our research experts and access our unique and modern facilities, resulting in collaborations that are highly ranked on traditional metrics and produce powerful narratives of innovation for Canada.
Achieving this vision requires a sustainable culture of excellence as well as a pattern of beneficial research outcomes and impacts. Our vision will be enabled by our people, facilities and collaborations, and how we collaborate and communicate as an organization both internally and externally. Guided by our Chief Science Officer and the President's Research Excellence Advisory Committee, and with input from experts across our organization, we will grow and sustain research excellence by:
- fostering the career development of world‑leading researchers
- increasing the exchange of ideas and providing time and opportunities for innovation
- enabling world‑leading research and innovation through facility renewals
- creating collaborative opportunities with world‑leading researchers in Canada and internationally
- creating space for the cross‑pollination of ideas by enhancing researcher interactions
- promoting multidisciplinary collaborations across the NRC, academia, industry and government
- communicating our results and the impacts of our work internally and externally, which will reinforce research excellence as a key success factor for the NRC, strengthen our collective pride in enabling this research and promote our success to Canadians and the world
A commitment to research excellence will ensure we produce high‑quality innovations as we achieve our strategic goals. It will further ensure the sustainability of the NRC as an engine for advancing research, technology and innovation.
A comprehensive structure of enabling teams and supporting business procedures—serving functions ranging from procurement to program development and from communications and contracting to IT enablement and support—rarely receive recognition equal to that of our research and innovation endeavours. But they are just as critical to our success. Continuing to improve these functions will be vital to advancing our research and innovation priorities over the next 5 years.
Over the last 5 years, we have made many changes that have helped us grow in the roles of partner and innovator. Initiatives like our Challenge programs and collaboration centres have allowed us to work more effectively with government, academia and industry, while providing more opportunities for scientific discoveries and technological advances.
In the spirit of continuous improvement, the 5 years ahead will see the next phase in our evolution, most notably the renewal of our infrastructure. Our national laboratories and facilities are foundational to our success and to the success of our partners. From astronomical observatories in British Columbia and photonics fabrication facilities in Ottawa to biomanufacturing facilities in Montreal and ocean tide simulators in Newfoundland and Labrador, the NRC manages and operates highly specialized equipment and infrastructure that have been instrumental in many scientific advances. We will make strategic investments enabled by an investment of nearly $1 billion from the Government of Canada to ensure our facilities remain on the leading edge of research and technology development. To deliver on this new investment, we will streamline our processes and leverage our increased authority in procurement to ensure we can operate at the speed of our partners.
Renewing our facilities will also help us reach our goal of reducing greenhouse gas emissions by 70% by 2030 and, in doing so, contribute to the Government of Canada's greening objectives. In addition to reducing the carbon footprint associated with the facilities themselves, we will purchase clean electricity as well as zero‑emission or hybrid vehicles for our light‑duty and executive fleet. Infrastructure renewal also provides an opportunity to make our physical spaces more inclusive and accessible, identify barriers and align design decisions with the Accessible Canada Act .
Finally, our ongoing investments in IT will ensure a strong and secure cyber environment, allowing us to work efficiently with government and businesses because they will trust in the security of our processes. We will incorporate automation and other digital tools into our internal operations, allowing us to move at the speed of business in our industrial partnerships. On the research front, we will renew our facilities with digital-powered tools and solutions that expand our capabilities and allow us to accelerate the speed at which we conduct research and innovation with our partners and for our clients.
Guided by the collective vision captured in our 2024–2029 strategic plan, we will forge ahead to achieve our goals and objectives. Because there is more than one path to success, our plan allows us the flexibility to adapt to unforeseen circumstances and opportunities that may emerge over the next 5 years. Whatever those may be, the plan will continue to guide us forward to better support our clients and partners and foster a more prosperous and innovative Canada.
We are also aware of risks that have the potential to affect our operations and the impact of our work. The escalation of international tensions and the potential increase of malicious cyber activity require increased attention on both research security and cyber security. Increased global competition for research talent and an aging population reinforce the importance of progressive human resources practices and proactive succession planning. We will also see new opportunities emerge. While our plan provides us with the flexibility to adapt, we will undergo periodic assessments to remain focused on important areas and aligned with Canadian priorities.
At the end of the plan's 5‑year period, we will consider success as having results and impacts under each of our goals, as well as having contributed to the following outcomes for Canada:
- successful transition to a prosperous, productive and competitive green economy and improved climate resilience
- connected biologics, biomanufacturing and distributed care industries that are competitive, ready to address emerging health priorities and grow to industrial scale
- improved competitiveness through the commercialization and adoption of digital and quantum technologies
- continued access to world‑class facilities and infrastructure for astronomy and measurement sciences
Over 5 years, success will also be measured by having upheld our values and grown stronger as an organization and as a partner for Canadian innovation. We will have seen growth in the areas of inclusive innovation, research excellence and organizational excellence, and we will have fostered a culture of the highest standard in health and safety.
Through our research and innovation activities, we will also have helped secure and grow Canada's sustainable industrial economy, make Canadian society and the economy more resilient, and put the country on a path for increased prosperity.
From: National Research Council Canada
Statement from the NRC – continued support for clients and collaborators during COVID-19
Statement from the National Research Council of Canada – continued support for clients and collaborators during COVID-19
April 9, 2020 – Ottawa, Ontario – As Canada and the world continue to deal with the COVID-19 pandemic, the National Research Council's (NRC) top priority is to protect the health and safety of our employees, colleagues, and stakeholders while supporting clients and collaborators. The NRC is committed to helping Canada and stakeholders during this time of need. We are prioritizing our pandemic response, mandated by the Prime Minister, to address urgent COVID-19 challenges and support Canadian businesses who are developing solutions. Please visit our website for more information and to contact us should you require our services.
In an effort to limit the spread of the virus, 90 percent of NRC staff have transitioned to teleworking. Employees who remain onsite are working on COVID-related programs, and performing critical tasks such as ensuring our buildings are secure, monitoring equipment and facilities, keeping systems running safely, and receiving essential shipments at loading docks on reduced hours. The NRC remains committed to all of our clients and collaborators, therefore we will continue to carefully consider which facilities need to remain open and which projects are critical to support Canada and Canadians during this difficult time.
NRC representatives will be reaching out to clients and collaborators directly, to understand their challenges and how we can best support them. We also continue to be available to discuss future projects.
For questions about payment terms, clients and collaborators are invited to contact the NRC's account receivable team at 1-866-545-1195 or [email protected] .
Media Relations National Research Council of Canada 613-991-1431 1-855-282-1637 [email protected]
We use cookies to understand how you use our site and to improve your experience. This includes personalizing content and advertising. To learn more, click here . By continuing to use our site, you accept our use of cookies, revised Privacy Policy and Terms of Service .

New to Zacks? Get started here.
Member Sign In
Don't Know Your Password?

- Zacks #1 Rank
- Zacks Industry Rank
- Zacks Sector Rank
- Equity Research
- Mutual Funds
- Mutual Fund Screener
- ETF Screener
- Earnings Calendar
- Earnings Releases
- Earnings ESP
- Earnings ESP Filter
- Stock Screener
- Premium Screens
- Basic Screens
- Thematic Screens
- Research Wizard
- Personal Finance
- Money Management
- Retirement Planning
- Tax Information
- My Portfolio
- Create Portfolio
- Style Scores
- Testimonials
- Zacks.com Tutorial
Services Overview
- Zacks Ultimate
- Zacks Investor Collection
- Zacks Premium
Investor Services
- ETF Investor
- Home Run Investor
- Income Investor
- Stocks Under $10
- Value Investor
- Top 10 Stocks
Other Services
- Method for Trading
- Zacks Confidential
Trading Services
- Black Box Trader
- Counterstrike
- Headline Trader
- Insider Trader
- Large-Cap Trader
- Options Trader
- Short Sell List
- Surprise Trader
- Alternative Energy

You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK . If you do not, click Cancel.
Image: Bigstock
Walgreens (WBA) Partners With BARDA to Boost Clinical Research
Walgreens Boots Alliance ( WBA Quick Quote WBA - Free Report ) recently announced a strategic partnership with BARDA (Biomedical Advanced Research and Development Authority) as part of the D-COHRe (Decentralized Clinical Operations for Healthcare and Research) program. The first-of-its-kind collaboration, valued at up to $100 million, intends to address barriers to decentralized clinical trial access and conduct trials over five years.
Walgreens is dedicated to making decentralized clinical research models more efficient and accessible to the U.S. population during their routine healthcare journey and public health emergencies.
News in Detail
BARDA, part of the Administration for Strategic Preparedness and Response in the U.S. Department of Health and Human Services, designed the D-COHRe initiative to bolster U.S. decentralized clinical research capabilities. The program aims to support the development of FDA-regulated products, enhance clinical innovation to execute more efficient and relevant clinical research and evaluate other medical countermeasures in real-world environments that may be used in a public health emergency.
The Walgreens-BARDA partnership will leverage Walgreens’ clinical trial ecosystem, which has proven to be effective in making clinical trials more accessible and representative of the U.S. population. Since its launch in 2022, it has reached more than five million patients to potentially recruit into clinical trials. The company has consistently met recruitment goals and continuously surpassed national averages for recruiting diverse clinical trial participants for its sponsor-led clinical trials.

Walgreens activates a comprehensive approach to participant recruitment, utilizing its physical footprint and decentralized clinical trial platform to engage patients and potential patients where it’s most convenient for them. This positions it as the ideal partner to help enhance decentralized clinical research to validate, pilot and implement new products, technologies and approaches for remote and/or decentralized use during a public health emergency, including immunizations, diagnostics and treatments.
Nearly 80% of trials fail to meet their enrollment goals in the stated timeframes, often contributing to billions of dollars in delays annually. With only 5% of the U.S. population participating in clinical trials, there is a clear need to increase access and representation in clinical research. Walgreens’ spokesperson expressed delight in continuing the partnership with BARDA to strengthen U.S. clinical research through a decentralized model in community pharmacies.
Industry Prospects
Based on a report by Precision Business Insights, the decentralized clinical trial market was valued at $1.68 billion in 2022 and is expected to witness a CAGR of 9.8% up to 2029.
The demand for accurate and high-quality data, reduced travel requirements and fewer time-consuming in-patient visits are the major factors driving the market growth. Moreover, the adoption of artificial intelligence tools for clinical trials is a major recent trend in the market.
Other Notable Developments
In July 2024, Walgreens was awarded a project valued at up to $25 million through the Rapid Response Partnership Vehicle, a Consortium funded by BARDA, to conduct a Phase IV observational clinical study. The study will focus on assessing Correlates of Protection, known as responses to a vaccine that predict how well a vaccinated person will be protected from future infections, using COVID-19 vaccine data.
Price Performance
In the past year, WBA shares have plunged 58.9% compared with the industry ’s fall of 23.7%.
Zacks Rank and Key Picks
Walgreens currently carries a Zacks Rank #5 (Strong Sell).
Some better-ranked stocks in the broader medical space are Masimo ( MASI Quick Quote MASI - Free Report ) , Boston Scientific ( BSX Quick Quote BSX - Free Report ) and Myriad Genetics ( MYGN Quick Quote MYGN - Free Report ) . While Masimo sports a Zacks Rank #1 (Strong Buy) at present, Boston Scientific and Myriad Genetics each carry a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks Rank #1 stocks here .
Masimo shares have risen 10.8% in the past year. Estimates for the company’s earnings have increased from $3.63 to $3.83 in 2024 and from $3.97 to $4.20 in 2025 in the past 30 days.
MASI’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 14.6%. In the last reported quarter, it posted an earnings surprise of 11.7%.
Estimates for Boston Scientific’s 2024 earnings per share have moved to $2.40 from $2.32 in the past 30 days. Shares of the company have rallied 56% in the past year compared with the industry’s rise of 11.1%.
BSX’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 7.2%. In the last reported quarter, it delivered an earnings surprise of 6.9%.
Estimates for Myriad Genetics’ 2024 earnings have moved to 5 cents from 3 cents per share in the past 30 days. Shares of the company have improved 59.3% in the past year against the industry’s 3.1% fall.
MYGN’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 213.4%. In the last reported quarter, it delivered an earnings surprise of a staggering 600%.
See More Zacks Research for These Tickers
Normally $25 each - click below to receive one report free:.
Boston Scientific Corporation (BSX) - free report >>
Masimo Corporation (MASI) - free report >>
Myriad Genetics, Inc. (MYGN) - free report >>
Walgreens Boots Alliance, Inc. (WBA) - free report >>
Published in
This file is used for Yahoo remarketing pixel add
Due to inactivity, you will be signed out in approximately:
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Biden Economy
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds Rates
- Top Mutual Funds
- Options: Highest Open Interest
- Options: Highest Implied Volatility
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Stock Comparison
- Advanced Chart
- Currency Converter
- Credit Cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Credit Card Offers
- Best Free Checking
- Student Loans
- Personal Loans
- Car insurance
- Mortgage Refinancing
- Mortgage Calculator
- Morning Brief
- Market Domination
- Market Domination Overtime
- Asking for a Trend
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Financial Freestyle
- Capitol Gains
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Walgreens and barda form strategic partnership to strengthen decentralized clinical trials model and reporting.
This first-of-its-kind collaboration between Walgreens and U.S. government aims to address barriers in decentralized clinical trial access and conduct trials over a five-year period, valued up to $100 million for the D-COHRe program
DEERFIELD, Ill., August 19, 2024 --( BUSINESS WIRE )--Walgreens and the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) in the U.S. Department of Health and Human Services (HHS), today announced a strategic partnership to increase innovation in decentralized clinical trials as part of the Decentralized Clinical Operations for Healthcare and Research ( D-COHRe ) program. The program is designed to strengthen U.S. decentralized clinical research capabilities to support development of U.S. Food and Drug Administration (FDA)-regulated products, enhance clinical innovation to execute more efficient and relevant clinical research and evaluate other medical countermeasures in real world environments that may be used in a public health emergency.
The partnership will utilize Walgreens’s clinical trial ecosystem, which has proven effective in making clinical trials more accessible and representative of the U.S. population and has reached more than five million patients to potentially recruit into clinical trials since its launch in 2022.
Walgreens is committed to making decentralized clinical research models more efficient and accessible to the U.S. population during their routine healthcare journey and during public health emergencies. Walgreens has consistently met recruitment goals and has continuously surpassed national averages for recruiting diverse clinical trial participants for its sponsor-led clinical trials. Walgreens successfully activates a comprehensive approach to participant recruitment that utilizes its physical footprint and its decentralized clinical trial platform to engage patients and potential patients where it’s most convenient for them. This positions Walgreens as the ideal partner to help enhance decentralized clinical research to validate, pilot and implement new products, technologies and approach for remote and/or decentralized use during a public health emergency, including immunizations, diagnostics, and treatments.
"It is a privilege to continue our partnership with BARDA to strengthen clinical research in the U.S. through a decentralized model in a community pharmacy setting like Walgreens," said Ramita Tandon, chief clinical trials officer at Walgreens. "Our network of community pharmacies and our compliant and secure clinical trial platform enables us to pioneer a comprehensive solution to make clinical research an integral part of a patient’s healthcare journey, especially when it is most critical for the well-being of our country, during a public health emergency."
Nearly 80% of trials fail to meet their enrollment goals in the stated timeframes, often contributing to billions of dollars in delays annually. With only 5% of the U.S. population participating in clinical trials, there is a clear need to increase access and representation in clinical research. Walgreens is committed to working with BARDA to help make decentralized clinical trials more accessible and representative of the U.S. population.
Walgreens is also partnering with BARDA on a Phase IV observational COVID-19 trial to enhance U.S. public health preparedness through the Walgreens community pharmacy network.
About Walgreens:
Walgreens ( http://www.walgreens.com ) is included in the U.S. Retail Pharmacy and U.S. Healthcare segments of Walgreens Boots Alliance, Inc. (Nasdaq: WBA), an integrated healthcare, pharmacy and retail leader. True to its purpose of "more joyful lives through better health," Walgreens has a more than 120-year heritage of caring for communities and providing trusted pharmacy services, and today is playing a greater role as an independent partner of choice offering healthcare services that improve care, lower costs, and help patients. Operating nearly 9,000 retail locations across the U.S. and Puerto Rico, Walgreens is proud to serve nearly 9 million customers and patients daily. The company’s pharmacists are playing a more critical role in healthcare than ever before, providing a wide range of pharmacy and healthcare services, including those that drive equitable access to care for some of the nation’s most underserved populations. Walgreens offers customers and patients a true omnichannel experience, with fully integrated physical and digital platforms designed to deliver high-quality products and healthcare services. Within the U.S. Healthcare segment, Walgreens portfolio also includes businesses in primary care, multi-specialty, post-acute care, urgent care, specialty pharmacy services, population health and provider enablement.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240819166725/en/
Walgreens Carmen Lopez [email protected]
- Audit and Assurance
- Business Succession Plan
- Business Transformation
- Cloud Services
- Consulting Services
- CRM and ERP Products
- CRM Services
- Cybersecurity
- Data and Analytics
- ERP Services
- Forensic and Valuation
- Governance, Risk and Compliance
- HEADSTART Implementations
- Human Capital Management and Payroll
- Insurance Services
- Internal Audit
- Investment Banking
- Lender Services
- IT and Security Managed Services
- Marketing and Communication
- Modern Workplace
- Outsourced Accounting
- Regulatory, Quality and Compliance
- Retirement Plan Services
- Site Selection and Incentives
- Spend Management
- Tax Services
- Transaction Advisory
- Wealth Management
- Workforce Risk Management

How should B2B brands respond to the latest LinkedIn changes?

Navigating the Biopharma Finance & Accounting Tech Landscape from Pre-Clinical to Commercial Success

Sikich Corporate Finance Serves as Exclusive Sell-Side Advisor to River Source Logistics in Acquisition by ShipCalm
- Construction and Real Estate
- Discrete Manufacturing
- Federal Government
- Financial Services
- Government Contractors
- Industrial Equipment Manufacturing
- Life Sciences
- Manufacturing and Distribution
- Not-for-Profit
- Professional Services
- Rolled Products Manufacturing
- State and Local Government
- Title IV Audit and Consulting
- Distribution and Supply Chain
- Audit & Assurance
- Forensic & Valuation
- Human Capital Management & Payroll
- IT and Managed Services
- Marketing and Communications
- Regulatory, Quality & Compliance
- Site Selection & Incentives
- Construction & Real Estate
- Not-for-profit
- Process Manufacturing
- Rolled Products
- State & Local Government
- Title IV Audit & Consulting
- Distribution & Supply Chain
- Lessons from Leadership

The biopharma industry is both exciting and challenging, especially when it comes to handling finance and accounting as companies move from pre-clinical to commercial stages. As your company grows, your financial needs become more complex. It’s important to understand the key tech considerations at each stage of your journey. Whether you’re just getting started or gearing up for a commercial launch, having the right tools and strategies is crucial for success.
To gain deeper insights into these topics, I encourage you to watch our joint on-demand video with Auxilius hosted by industry experts. The webinar covers detailed discussions on finance and accounting tech decisions tailored to different stages of biopharma companies.
The Pre-Clinical Stage: Laying the Groundwork
In the early days of a biopharma company, the focus is more on innovation and proving concepts. At this stage, most companies rely on simple and cost-effective financial solutions like QuickBooks. It’s familiar, inexpensive, and gets the job done. However, as you edge closer to significant milestones like IPOs, the limitations of such basic systems become evident. QuickBooks works well for initial needs due to its simplicity and ease of use, but as your operations scale, you require more robust controls and comprehensive reporting capabilities. This shift often necessitates moving to a more sophisticated system like NetSuite or Microsoft D365 to manage the increased complexity.
The Clinical Stage: Scaling and Managing Complexity
As your company progresses to the clinical stage, financial management becomes more intricate. You’re dealing with multiple clinical trials, significant R&D expenses, and rigorous regulatory requirements. A basic financial system can no longer meet your needs efficiently. This is where ERP systems like NetSuite come into play. This system provides advanced functionalities such as multi-entity management, real-time financial reporting, and robust compliance features, which are essential as you prepare for commercialization.
Transitioning to NetSuite
Moving from QuickBooks to a full-fledged ERP system is a big leap, but it’s crucial for managing the financial complexities of clinical trials. These ERP systems offer tools for accurate accruals, budget management, and vendor accountability, ensuring compliance with regulatory standards and reducing the risk of errors.
Example: Handling Clinical Trial Financial Management (CTFM) Manual processes for managing clinical trial finances can lead to inefficiencies and inaccuracies. An ERP system like NetSuite automates these processes, providing tools for precise financial tracking and reporting, which is vital as the stakes increase.
The Commercial Stage: Optimizing Operations
As your company transitions to the commercial stage, the focus shifts to optimizing operations. You’re now generating revenue and managing a global supply chain. Your ERP system must handle increased complexity and scale effectively. Key capabilities at this stage include inventory and supply chain management, revenue recognition, and global compliance.
Key Capabilities for Commercial Success
- Inventory and Supply Chain Management : Efficiently managing inventory and coordinating with CMOs and 3PLs is vital.
- Revenue Recognition : Accurately calculating and reporting revenue, including handling gross-to-net adjustments, becomes crucial.
- Global Compliance : Ensuring compliance with international regulations if you’re operating across multiple countries.
Example: Using NetSuite for Commercial Operations NetSuite’s capabilities extend to managing complex inventory and supply chain needs, making it an ideal solution for biopharma companies scaling their operations. Its integration capabilities with third-party logistics providers ensure real-time visibility and efficient management of your supply chain.
Auxilius: Bridging the Gaps in Clinical Financial Management
Even with a robust ERP system, gaps remain, particularly in clinical financial management. Auxilius is designed specifically for life sciences, helping companies streamline period close processes, manage clinical R&D accruals, and maintain accurate budgets.
Why Auxilius Matters
Auxilius addresses the unique challenges faced by biopharma companies, providing tools tailored to the intricacies of clinical financial management. By integrating with your existing ERP system, Auxilius enhances your ability to manage clinical trial finances efficiently and accurately.
Example: Streamlining Clinical Trial Accruals Manual processes for managing clinical trial accruals are prone to errors and inefficiencies. Auxilius automates these processes, ensuring accurate and timely financial reporting. This not only reduces the risk of compliance issues but also frees up your finance team to focus on more strategic tasks.
Strategic Considerations and Future Planning
Regularly assessing your tech stack is critical to ensure it meets your evolving needs. Conducting an annual review of your systems can help identify gaps and opportunities for improvement. This proactive approach allows you to stay ahead of the curve and adapt to changes in the industry.
Questions to Ask During Your Tech Review
- Can our current systems handle our projected growth?
- Are we leveraging all the functionalities of our ERP system?
- Do we need additional point solutions to address specific needs?
- How well are our systems integrated?
- Are we prepared for future regulatory changes?
Navigating the biopharma finance and accounting tech landscape is a journey that requires careful planning and strategic decisions. By understanding the specific needs at each stage of your company’s growth and leveraging the right tools, you can ensure financial stability and compliance, paving the way for successful commercialization. Keep evaluating your tech stack, stay informed about industry advancements, and don’t hesitate to seek expert advice to keep your company on the path to success.
This publication contains general information only and Sikich is not, by means of this publication, rendering accounting, business, financial, investment, legal, tax, or any other professional advice or services. This publication is not a substitute for such professional advice or services, nor should you use it as a basis for any decision, action or omission that may affect you or your business. Before making any decision, taking any action or omitting an action that may affect you or your business, you should consult a qualified professional advisor. In addition, this publication may contain certain content generated by an artificial intelligence (AI) language model. You acknowledge that Sikich shall not be responsible for any loss sustained by you or any person who relies on this publication.
About the Author
Jim Ingram is a business development representative for Sikich. He specializes in using technology like NetSuite and Microsoft Dynamics to help companies improve their business outcomes. Jim works closely with Clinical Research Organizations (CROs) and Life Science Organizations to improve and streamline technology systems.
Sign up for Insights
Join 14,000+ Business executives and decision makers.
Latest Insights
Marketing & Communications
How should B2B brands respond to the latest LinkedIn changes...
August 20, 2024
Earlier this year, LinkedIn made two major changes that impact how companies reach their target audiences on LinkedIn. A change to the LinkedIn a...
Navigating the Biopharma Finance & Accounting Tech Lands...
August 19, 2024
The biopharma industry is both exciting and challenging, especially when it comes to handling finance and accounting as companies move from pre-clini...
News Release
Sikich Corporate Finance Serves as Exclusive Sell-Side Advis...
August 16, 2024
CHICAGO – August 16, 2024 – Sikich Corporate Finance, a leading investment bank for middle market companies and a wholly owned subsidiary of Siki...
Is AI making LinkedIn the hub of B2B customer engagement?
The traditional model of B2B digital lead generation puts a company's website at the center of the buyer journey. A company creates high-value conten...
Managing Organizational Complexity in BioTech: NetSuite’s ...
BioTech companies often have multiple subsidiaries and operations across different regions. They engage in joint ventures, acquisitions, strategic al...
Sikich On Demand
On Demand – Strategic Tax Planning for Businesses: Pre...
August 15, 2024
https://youtu.be/i8aTxCgP210?si=iCEtDGOT-neoB0lB Tune into our webinar, where Sikich tax experts highlight TCJA provisions that are scheduled to...
If You Want Your ERP Implementation to Be Successful, Take T...
The process of choosing and implementing a new ERP solution can be long and challenging. There are plenty of moving parts to keep track of and divers...
Transaction Advisory Services
Buy-Side Deliverable Options in Private Equity Reporting
August 14, 2024
Financial reporting due diligence guides buyers through complex investments, putting you on the right path to uncover the true value of potential acq...
Manufacturing
What’s Driving Manufacturers’ Plans for Tech Investments...
The U.S. manufacturing sector has been through the wringer over the past few years. COVID, supply chain disruption, extreme weather events, and globa...
The Implications of the TCJA Expiration for Section 1202
August 13, 2024
Join us on August 27 for an in-depth review of the TCJA individual and estate tax planning provisions, upcoming sunsets, and crucial tax plannin...
- Privacy Overview
- Strictly Necessary Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.

IMAGES
COMMENTS
Updates to the Plan. The NIMH Strategic Plan for Research is a living document, which means it is updated regularly to keep pace with ever-evolving scientific approaches and research priorities that can lead to new discovery. The most recent update was published in July 2021.
Research Strategic Plan. In 2019, the Department of Medicine invested considerable effort and resources to devising a strategic plan that will provide a roadmap for our research mission today and into the future. This work was guided by a Research Planning Committee that convened throughout the first half of 2019, reviewing the current state of ...
Introduction. Strategic planning is a forward-looking process to set priorities, focus resources on common goals, strengthen operations and maintain vitality of an organization; it is "a deliberative, disciplined approach to producing fundamental decisions and actions that shape and guide what an organization or other entity is, what it does, and why" ().
Strategic planning provides the structure to make day-to-day decisions that follow a larger vision, creates a direction for your practice, and maximizes your options for influencing your environment. In oncology practice, where dramatic changes in reimbursement, technology, and the marketplace are just a few of the driving forces, "the future ...
Building on this effort in 2020, Yale School of Medicine has developed a strategic plan for scientific research that aligns with the broader plan and builds on key strengths in the school. ... The School of Medicine has identified three cross-cutting themes that will support basic, translational, and clinical researchers across the organization.
In 2005, NIAID's Division of Clinical Research (DCR), to help meet its goals and objectives, implemented the Kaplan-Norton (K-N) strategy management paradigm . Strategy management is an overarching process incorporating strategic planning, monitoring, analysis, and assessment of all the elements (including organizational structure, core ...
Updates to the Plan. The NIMH Strategic Plan for Research is a living document, which means it is updated regularly to keep pace with ever-evolving scientific approaches and research priorities that can lead to new discovery. The most recent update was published in May 2024.
NIH-Wide Strategic Plan for Fiscal Years 2021-2025. i. Director's Message. To the American People, As our nation's biomedical research agency, the National Institutes of Health (NIH) ... Phoenix Epidemiology and Clinical Research Branch in Phoenix, Arizona. Scientists in the Intramural Research Program include an estimated 1,200
developed a strategic plan for scientific research that aligns with the broader plan and builds on key strengths in the school. The strategic planning process began with one-on-one interviews with more than 80 faculty members who were identified by chairs, center directors, and deans as the future leaders of YSM. The
In October 2019, the Clinical Trials and Translational Research Advisory Committee (CTAC) of the National Cancer Institute (NCI) established an ad hoc Strategic Planning Working Group charged with assessing NCI's strategic vision for its clinical trials system for 2030 and beyond and making recommendations to achieve that vision.
In developing this Strategic Plan, we have focused on the areas of science in which the needs are greatest, and for which we believe NINR-supported science can have the largest impact. The Plan refects not only the long-standing focus areas of nursing science, but also areas in which nurse scientists can use their expertise in clinical research and
This strategic plan is driven by three guiding principles: Consider the complex intersection among multiple factors that afect the health of women. Include diverse populations of women in clinical research. Integrate perspectives from a diverse workforce of scientists with difering skills, knowledge, and experience.
The National Institute of Nursing Research 2022-2026 Strategic Plan ... Nurses interact with individuals and families more closely than other health professionals in the many clinical, community, and policy settings in which they work; thus, they have a deep understanding of the personal and societal factors that lead to health among some, and ...
The NIH FY 2021-2025 Strategic Plan to Advance Research on the Health and Well-being of Sexual and Gender Minorities builds upon the foundation of the previous NIH SGM Research Strategic Plan, pre-senting scientific themes and operational goals and objectives that. Karen L. Parker, Ph.D., M.S.W.
The plan creates a unified vision for the future with researchers, the public, and policymakers. To develop the 2023-2027 Strategic Plan, NIDCD solicited input from scientific experts, the National Deafness and Other Communication Disorders (NDCD) Advisory Council, NIDCD staff, and the public. See Appendix A for more details on the strategic ...
The importance of designing your clinical program with the end in mind, from First-in-Man through approval and commercialization. Perspectives: Investors vs. stakeholders vs. market. Balancing risk with investment opportunity. Understanding the specific landscape and market for your product. Thinking ahead without investing major resources.
The Strategic Plan's Leap Forward Research Challenges are goals that aim to redefine the science of minority health and health disparities. These goals include: Increase the overall proportion of participants from diverse populations included in NIH-funded clinical research to 40 percent by 2030 and within specific major disease categories.
Clinical research should not be a corollary in the strategic planning of health management, but rather it should be a primary component in the array of mid- to long-term goals, and also part of the investment plan. ... Platforms for clinical research include first multiple databases implemented in each main field of interest to systematically ...
Strategic Plan 2025-2029 Download Strategic Plan (PDF, X Mb) Download ... in well-controlled intervention research and what works when those interventions are implemented in real-world clinical or community settings. Our previous strategic plan identified a need for more research designed to answer questions that can facilitate rapid, data ...
The plan consists of Strategic Pillars - goals - that will improve health care delivery for patients throughout Northern California. These areas of emphasis include: Developing a regional system of care - reaching more patients in more places. Delivering an exceptional patient and care team experience - investing in patients and ...
This investigator met with our Connector team early in his grant planning process. His studies are now funded, and he's running them through our main Clinical Research Center at the Boston campus. But he is having a really hard time meeting his recruitment goals, so we are working with him to set up research support at our Bowdoin Street ...
Background: A structured process of Strategic Planning is critical to the organization success, including clinical laboratories. This process, often have limited participation to directors and ...
Clinical Research Question: Among intensive care (or acute or critical care) nurses, will mindfulness-based interventions and increased physical activity (or exercise interventions) decrease burnout syndrome (professional burnout) and increase job satisfaction (retention)?
However, historical clinical research, from which the industry draws insights for future studies, often lacks diverse data, perpetuating imbalances and leaving key patient populations disenfranchised.
Finally, the degree of uncertainty grows exponentially with the distance between the day of planning and the day of action, i.e., the higher the time horizon, the higher the uncertainty. Consequently, strategic planning is planning under uncertainty with many different alternatives that can occur. This is reflected by scenarios or simulations.
Strategic plan at a glance Research and innovation priorities Climate change and sustainability. ... Our new Clinical Trial Material Facility, the only public facility of its kind, will serve as a product development bridge for domestic production of vaccines compliant with good manufacturing practice (GMP) regulations and other biologics ...
Walgreens (WBA) and BARDA form a strategic partnership to bolster the decentralized clinical trial model. Walgreens (WBA) Partners With BARDA to Boost Clinical Research - August 20, 2024 - Zacks.com
DEERFIELD, Ill., August 19, 2024--Walgreens and the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) in the ...
Navigating the biopharma finance and accounting tech landscape is a journey that requires careful planning and strategic decisions. By understanding the specific needs at each stage of your company's growth and leveraging the right tools, you can ensure financial stability and compliance, paving the way for successful commercialization.
Horses can plan and strategise, new study shows. Getty Images. You can lead a horse to water and, it turns out, convince it to drink if the reward is great enough, researchers have found.