
ANDREW J. GOODBRED, MD, AND ROBERT C. LANGAN, MD
Am Fam Physician. 2023;108(6):554-561
Author disclosure: No relevant financial relationships.
Chronic kidney disease (CKD) affects approximately 15% of the U.S. population, and many people are unaware of their diagnosis. Screening may be considered for patients with cardiovascular disease, diabetes mellitus, hypertension, age 60 years and older, family history of kidney disease, previous acute kidney injury, or preeclampsia. Diagnosis and staging of CKD are based on estimated glomerular filtration rate (eGFR), excessive urinary albumin excretion, or evidence of kidney parenchymal damage lasting more than three months. eGFR should be determined using the CKD-EPI creatinine equation without the race variable. Risk calculators are available to estimate the risk of progression to end-stage renal disease. When possible, serum cystatin C should be measured to confirm eGFR in patients with CKD. Blood pressure should be maintained at less than 140/90 mm Hg, with a systolic blood pressure target of 120 mm Hg or less for patients tolerant of therapy, using an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. Sodium-glucose cotransporter-2 inhibitors and metformin should be considered in patients with CKD and type 2 diabetes who have not reached their glycemic goal. Intravenous iodinated contrast media temporarily reduces eGFR and should be avoided in patients with advanced CKD. Interdisciplinary management of patients with CKD is important for reducing morbidity and mortality, and patients at high risk of progression to end-stage renal disease should be referred to a nephrologist.
Chronic kidney disease (CKD) affects about 15% of the U.S. population; however, 9 out of 10 people do not know they have impaired renal function. 1 CKD is diagnosed in Black people three times as often as in White people. 1 , 2 CKD is more common in women than men, but men are more likely to progress to end-stage renal disease (ESRD). 1 , 2 CKD is more common in patients 60 years and older compared with younger patients, and more advanced disease is associated with an increased risk of cardiovascular disease and death. 1 , 3

Definition and Staging
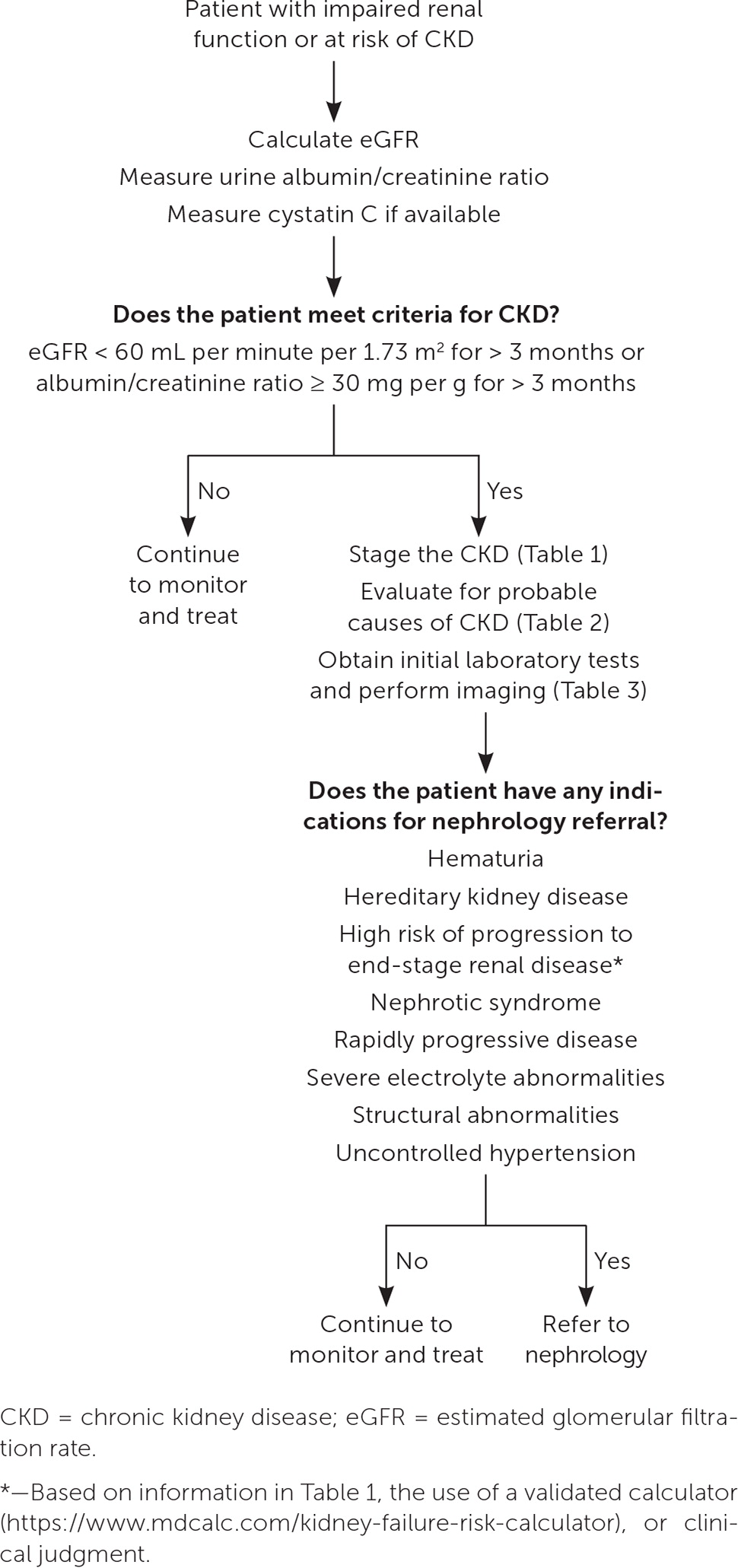
CKD is defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL per minute per 1.73 m 2 or markers of kidney damage, including functional or structural abnormalities such as albuminuria (albumin excretion rate of 30 mg or more daily or albumin/creatinine ratio of 30 mg per g or greater), abnormal urinalysis, and polycystic or dysplastic kidneys. CKD persists for more than three months. 4 The Kidney Disease: Improving Global Outcomes (KDIGO) staging of CKD is based on the eGFR category and the level of persistent albuminuria ( Table 1 ) . 5 High levels of proteinuria are associated with an increased risk of disease progression, even if the eGFR is normal. ESRD is defined as the need for renal replacement therapy or renal transplant. Risk calculators can help physicians identify patients with CKD at high risk of developing ESRD ( https://www.mdcalc.com/kidney-failure-risk-calculator ); however, it is unknown if these calculators improve the management of CKD. 3
The National Kidney Foundation/American Society of Nephrology Task Force recommends that physicians use the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation without the race variable included to calculate eGFR ( https://www.mdcalc.com/calc/3939/ckdepi-equations-glomerular-filtration-rate-gfr ) . This equation included diverse populations in its creation, is widely available, and performs well clinically. 6 Serum cystatin C, which is produced at a constant rate and is independent of race, should be used to confirm eGFR in patients with CKD; combining it in an enhanced equation with CKD-EPI or creatinine is more accurate than either alone. 6 Cystatin C levels may not be as accurate in acute kidney injury, inflammatory states, or thyroid dysfunction and may not be as widely available as the CKD-EPI creatinine equation. 7
Albuminuria should be quantified by a urine albumin/creatinine ratio. The urine albumin/creatinine ratio is preferred to a urine protein/creatinine ratio due to more widespread standardization and improved accuracy at lower levels of albuminuria. 5 Testing should be obtained from a first-morning sample or a 24-hour urine collection to improve accuracy. 5 , 8 An elevated urine a lbumin/creatinine ratio on two or more samples over at least three months indicates CKD because a transient increase in albumin/creatinine ratio may represent acute causes such as urinary tract infection, acute kidney injury, and systemic infection. 5 , 8
Screening and Indications
Most patients with CKD are asymptomatic. Symptoms are more common in advanced disease and may include fatigue, nausea, vomiting, anorexia, insomnia, and edema. 3 KDIGO recommends screening patients with cardiovascular disease, diabetes mellitus, or hypertension and those at high risk (i.e., age 60 years and older, family history of kidney disease, previous acute kidney injury, or preeclampsia) for CKD based on eGFR and albumin/creatinine ratio. 9 The U.S. Preventive Services Task Force is reviewing its CKD screening recommendation. 10 No randomized controlled trials have shown improved outcomes with screening of asymptomatic individuals. 3
The most common causes of CKD are diabetes (38%) and hypertension (26%). 11 Other causes can be divided into nephrotoxic medications; malignancy; and anatomic, autoimmune, genetic, infectious, metabolic, obstructive, and vascular processes ( Table 2 3 , 5 ) . More than one process may be present. A history and physical examination (including blood pressure and weight measurement) should be performed. The recommended initial laboratory and imaging tests for all patients with CKD are listed in Table 3 . 3 , 5 Testing for less common underlying conditions, such as autoimmune conditions or polycystic kidney disease, is based on the presumed diagnosis suggested by the history and physical examination. Indications for early referral to a nephrologist include hematuria, hereditary kidney disease, high risk of progression to ESRD, nephrotic syndrome, rapidly progressive disease, severe electrolyte abnormalities, structural abnormalities, or uncontrolled hypertension. 3 Figure 1 provides steps for the initial diagnosis, staging, and management of CKD. 3 , 5 , 12
Management and Prevention
Many strategies that have been recommended to limit the progression of CKD are also recommended to prevent CKD in patients with multiple risk factors. Implementing lifestyle interventions, avoiding nephrotoxic substances, and managing comorbid health conditions demonstrate benefits.
Evidence-based lifestyle interventions for the prevention and treatment of CKD include a diet low in sodium (less than 2,000 to 2,300 mg per day), a structured moderate-intensity exercise program of at least 150 minutes per week, and smoking cessation. 5 , 13 – 15 Dietary protein should be limited to 0.6 to 0.8 g per kg per day in patients with CKD stage 3 or 4 to reduce disease progression. 13 , 15 – 17 Resistance exercise and adequate caloric and micronutrient intake are recommended to decrease the risk of sarcopenia. 13 , 15 – 17 Plant-based diets have demonstrated benefits in the prevention and management of CKD because of reduced animal protein intake, increased consumption of anti-inflammatory phytonutrients, and their positive effect on controlling body weight, blood pressure, and blood glucose. 18 – 21
Patients engaging in strenuous endurance exercise should ensure adequate hydration to avoid dehydration-related kidney injury and rhabdomyolysis. 22 The use of nephrotoxic drugs should be limited or avoided where possible. 23 Many medications require dosing adjustment in patients with CKD due to the potential for direct renal insult or a change in their magnitude of effect in patients who have reduced renal function. 5 , 13 In addition to routine age-based vaccinations, a pneumococcal vaccination should be administered to patients between 19 and 64 years of age with ESRD and those on dialysis. 24 , 25
HYPERTENSION
In 2021, KDIGO’s updated guideline on the management of hypertension in patients with CKD recommended that lifestyle and medication interventions target a systolic blood pressure of 120 mm Hg or less if tolerated. 26 This recommendation is based on evidence from a high-quality randomized controlled trial showing that lowering systolic blood pressure below 120 mm Hg is associated with a reduction in cardiovascular events and all-cause mortality in patients who do not have diabetes but are at increased risk of cardiovascular events (e.g., known cardiovascular disease other than stroke, CKD with eGFR of 20 to 60 mL per minute per 1.73 m 2 excluding polycystic kidney disease, 10-year cardiovascular disease risk of greater than 15% based on Framingham risk score, or age 75 years or older). 26 , 27 The benefit of this more aggressive approach in patients with CKD stage 4 or 5, and in those with comorbid CKD and diabetes, is less certain when compared with the risk of adverse events such as acute kidney injury and electrolyte disturbance. 26 This contrasts with the 2019 U.S. Department of Veterans Affairs/Department of Defense guidelines, which recommend a target blood pressure of less than 140/90 mm Hg. 13 Patients with known cardiovascular disease and those at increased cardiovascular risk are most likely to benefit from more intensive management. 26 Patients with hypertension, diabetes, CKD, and moderate to severe albuminuria (A2 to A3) should be treated with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. 5 , 13 , 26 Both classes are preferred in patients without diabetes who have CKD and microalbuminuria due to a demonstrated reduction in progression to ESRD, with a goal of reaching the highest-tolerated dose to maximize benefit. 5 , 13 , 24 , 28
Finerenone (Kerendia), a nonsteroidal mineralocorticoid receptor antagonist that lowers the risk of disease progression and cardiovascular events when used in patients with CKD and type 2 diabetes, may be considered in patients with continued disease progression despite maximal medication therapy. 29 , 30 Discontinuation of renin-angiotensin-aldosterone system blockades in patients with CKD stages 4 or 5 is not routinely recommended; however, there is little supporting evidence that discontinuation leads to worse outcomes. 31 – 33 Other cardiovascular risk reduction strategies, such as the use of statins, should be used according to established guidelines. 34 Adults 50 years and older with CKD who are not on dialysis and those between 18 and 49 years of age with CKD and risk factors qualify for statin therapy. 34
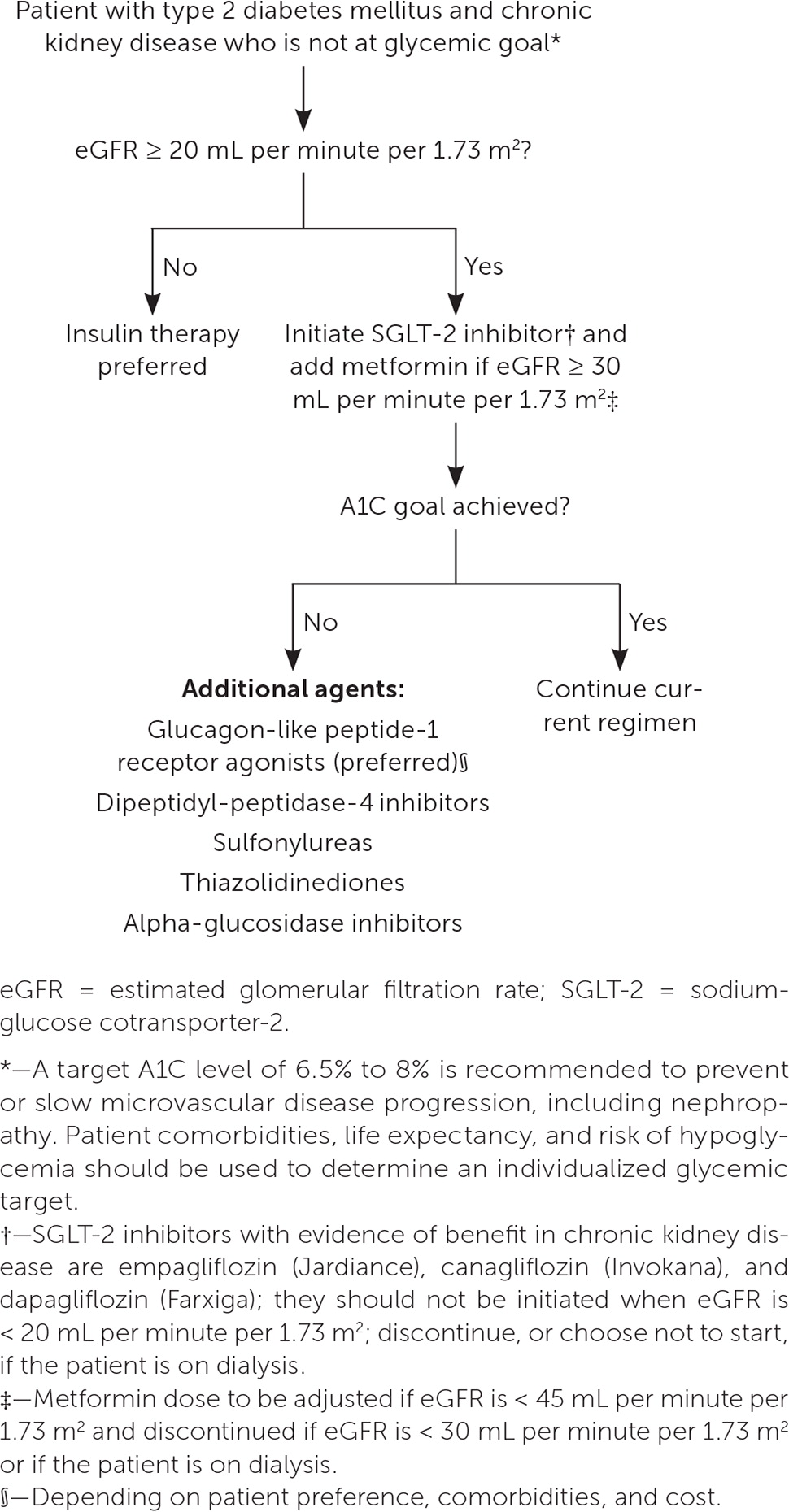
In patients with CKD and diabetes, a target A1C of 6.5% to 8% is recommended to prevent or slow microvascular disease progression, including nephropathy. 5 , 13 Patient comorbidities, life expectancy, and risk of hypoglycemia should be used to determine an individualized glycemic target. 5 , 35 Sodium-glucose cotransporter-2 (SGLT-2) inhibitors and metformin are recommended as first-line treatments in patients with CKD and type 2 diabetes who have not reached their glycemic goal. SGLT-2 inhibitors reduce renal disease progression and cardiovascular disease risk, independent of their effect on glycemic control, with risk reduction noted in patients with CKD who do not have diabetes. 35 – 38 Due to the risk of diabetic ketoacidosis, SGLT-2 inhibitors are contraindicated in patients with type 1 diabetes. 13 Metformin is recommended in CKD stages 1 to 3 with an eGFR of 30 mL per minute per 1.73 m 2 or greater, but it is contraindicated in stages 4 and 5. 33 , 35 Glucagon-like peptide-1 (GLP-1) receptor agonists also demonstrate protective effects against CKD progression, cardiovascular disease, and all-cause mortality. 13 , 35 , 39 SGLT-2 inhibitors and GLP-1 receptor agonists share the additional benefit of a very low risk of hypoglycemia. 5 , 38 – 40 Figure 2 outlines recommendations for glucose-lowering medications in patients with CKD and diabetes. 35
Serum hemoglobin should be measured at least once per year in patients with CKD stage 3 or greater because of the increased risk of anemia in CKD. 13 , 41 Detailed information on screening for and monitoring complications of CKD is listed in Table 4 . 5 , 13 , 41 , 42 Table 5 offers testing and management recommendations for complications of CKD. 3 , 5 , 13 , 41 , 42 Anemia may have several causes; therefore, a reticulocyte count and measurement of ferritin, transferrin saturation, folate, and vitamin B 12 levels are indicated in patients with anemia to confirm the etiology. 41 For patients requiring iron supplementation, deciding on the route of administration depends on the severity of iron deficiency, previous response to oral repletion, cost, and venous access. 41 Administration of erythropoiesis-stimulating therapy requires careful consideration of the potential reduction in blood transfusion and anemia symptoms relative to the risk of complications such as stroke, loss of vascular access, and hypertension. 41
Erythropoiesis-stimulating medications should not be initiated in patients with CKD and a starting hemoglobin level of more than 10 g per dL (100 g per L); a target of no more than 11.5 g per dL (115 g per L) is recommended. 13

BONE MINERAL DISORDERS
Beginning in CKD stage 3a, serum levels of calcium, phosphate, parathyroid hormone, and alkaline phosphatase should be obtained, with frequency determined by the CKD stage and previous results. 42 25-Hydroxyvitamin D testing is recommended in patients with CKD stage 3 and greater, with treatment of insufficiency or deficiency. 42 Bone mineral density testing with dual energy x-ray absorptiometry is recommended in patients with CKD stage 3 and greater and biochemical evidence of bone mineral disease, a history of fragility fracture, or an elevated risk of osteoporosis as defined by U.S. Preventive Services Task Force guidelines, provided that results would change management. 42 Treatment of osteoporosis in patients with CKD is similar to that of the general population. 42 , 43
Calcitriol, active vitamin D analogues, and calcimimetics should not be given to lower elevated parathyroid hormone levels in patients with CKD stages 3 or 4 due to unclear benefits to bone health and an increased risk of abnormalities in calcium and phosphorous levels. 13 , 42 They may have a role in the treatment of secondary hyperparathyroidism in patients with CKD stage 5 or ESRD. 42
USE OF CONTRAST MEDIA IN PATIENTS WITH CKD
Intravenous iodinated contrast media temporarily reduces eGFR and should be used selectively in patients with CKD. Preprocedure and postprocedure isotonic intravenous fluids should be used for nonurgent imaging studies to decrease the risk of worsened kidney function through improvement in renal perfusion and contrast dilution. Intravenous iodinated contrast media is not recommended in patients with diabetes and CKD stage 3a or in patients without diabetes and CKD stage 3b or greater. 13 If contrast studies are considered in patients with CKD stages 4 or 5, a nephrologist should be consulted for management recommendations. No evidence suggests that holding angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, or other medications for contrast studies decreases the risk of kidney injury. 44 Evidence is insufficient to demonstrate a clear protective effect with preprocedural N -acetylcysteine use, and there are potential adverse effects on myocardial and coagulation function when given at higher intravenous doses. 13 , 44 Renal replacement therapy should not be used routinely as prophylaxis against contrast-induced injury. 13 Newer generation gadolinium-based contrast media can be safely used in patients with CKD. 45
Indications for Referral
Early collaboration between family physicians and nephrologists allows for an interdisciplinary approach to patient education, detection, and management of complications, and planning for the progression of renal disease.
This article updates previous articles on this topic by Gaitonde, et al. 46 ; Rivera, et al. 47 ; and Baumgarten and Gehr . 48
Data Sources: A PubMed search was completed in Clinical Queries using the key terms chronic kidney disease and management. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The Cochrane database, DynaMedex, and Essential Evidence Plus were also searched. If studies used race or gender as patient categories, but did not define how these categories were assigned, they were not included in the final review. Studies that addressed concerns with including race in diagnosis and management are explicitly mentioned in the article. Search dates: January 4, 2023; May 12, 2023; and September 19, 2023.
- Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2021. Accessed February 3, 2023. https://nccd.cdc.gov/CKD/Documents/Chronic-Kidney-Disease-in-the-US-2021-h.pdf
Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 suppl 1):A6-A7.
Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294-1304.
Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes consensus conference. Kidney Int. 2020;97(6):1117-1129.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150.
Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79(2):268-288.e1.
Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine. Am J Kidney Dis. 2008;51(3):395-406.
Witte EC, Lambers Heerspink HJ, de Zeeuw D, et al. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20(2):436-443.
Shlipak MG, Tummalapalli SL, Boulware LE, et al.; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47.
U.S. Preventive Services Task Force. Chronic kidney disease: screening. Updated July 13, 2023. Accessed September 21, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/chronic-kidney-disease-screening
Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet. 2017;389(10075):1238-1252.
Tangri N, Grams ME, Levey AS, et al.; CKD Prognosis Consortium. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis [published correction appears in JAMA . 2016; 315(8): 822]. JAMA. 2016;315(2):164-174.
U.S. Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of chronic kidney disease. 2019. Accessed September 21, 2023. https://www.healthquality.va.gov/guidelines/CD/ckd
Espeland MA, Gaussoin SA, Bahnson J, et al. Impact of an 8-year intensive lifestyle intervention on an index of multimorbidity. J Am Geriatr Soc. 2020;68(10):2249-2256.
Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD [published correction appears in Am J Kidney Dis . 2021; 77(2): 308]. Am J Kidney Dis. 2020;76(3 suppl 1):S1-S107.
Hahn D, Hodson EM, Fouque D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev. 2020(10):CD001892.
Rhee CM, Ahmadi SF, Kovesdy CP, et al. Low-protein diet for conservative management of chronic kidney disease. J Cachexia Sarcopenia Muscle. 2018;9(2):235-245.
Chauveau P, Koppe L, Combe C, et al. Vegetarian diets and chronic kidney disease. Nephrol Dial Transplant. 2019;34(2):199-207.
Joshi S, Hashmi S, Shah S, et al. Plant-based diets for prevention and management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2020;29(1):16-21.
Yuzbashian E, Asghari G, Mirmiran P, et al. Associations of dietary macronutrients with glomerular filtration rate and kidney dysfunction: Tehran lipid and glucose study. J Nephrol. 2015;28(2):173-180.
Oosterwijk MM, Soedamah-Muthu SS, Geleijnse JM, et al. High dietary intake of vegetable protein is associated with lower prevalence of renal function impairment. Kidney Int Rep. 2019;4(5):710-719.
Cabral BMI, Edding SN, Portocarrero JP, et al. Rhabdomyolysis. Dis Mon. 2020;66(8):101015.
Perazella MA. Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol. 2018;13(12):1897-1908.
Murthy N, Wodi AP, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):229-233.
Centers for Disease Control and Prevention. Pneumococcal vaccination: summary of who and when to vaccinate. February 13, 2023. Accessed June 1, 2023. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1-S87.
Wright JT, Williamson JD, Whelton PK, et al.; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control [published correction appears in N Engl J Med . 2017; 377(25): 2506]. N Engl J Med. 2015;373(22):2103-2116.
Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD. Am J Kidney Dis. 2016;67(5):728-741.
Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229.
Pitt B, Filippatos G, Agarwal R, et al.; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252-2263.
Fu EL, Evans M, Clase CM, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32(2):424-435.
Bhandari S, Mehta S, Khwaja A, et al.; STOP ACEi Trial Investigators. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387(22):2021-2032.
ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2023;46(suppl 1):S140-S157.
Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD. Kidney Int. 2014;85(6):1303-1309.
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1-S127.
Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
Herrington WG, Staplin N, Wanner C, et al.; The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127.
Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2019;21(5):1237-1250.
Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes [published correction appears in Lancet Diabetes Endocrinol . 2020; 8(3): e2]. Lancet Diabetes Endocrinol. 2019;7(10):776-785.
Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279-335.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder [published correction appears in Kidney Int Suppl . 2017; 7(3): e1]. Kidney Int Suppl. 2017;7(1):1-59.
Hara T, Hijikata Y, Matsubara Y, et al. Pharmacological interventions versus placebo, no treatment or usual care for osteoporosis in people with CKD stages 3-5D. Cochrane Database Syst Rev. 2021(7):CD013424.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138.
Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2021;298(1):28-35.
Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2017;96(12):776-783.
Rivera JA, O’Hare AM, Harper GM. Update on the management of chronic kidney disease. Am Fam Physician. 2012;86(8):749-754.
Baumgarten M, Gehr T. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2011;84(10):1138-1148.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2023 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
