Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 August 2023

The recovery of European freshwater biodiversity has come to a halt
- Peter Haase ORCID: orcid.org/0000-0002-9340-0438 1 , 2 na1 ,
- Diana E. Bowler ORCID: orcid.org/0000-0002-7775-1668 3 , 4 , 5 ,
- Nathan J. Baker ORCID: orcid.org/0000-0001-7948-106X 1 , 6 ,
- Núria Bonada 7 ,
- Sami Domisch ORCID: orcid.org/0000-0002-8127-9335 8 ,
- Jaime R. Garcia Marquez 8 ,
- Jani Heino ORCID: orcid.org/0000-0003-1235-6613 9 ,
- Daniel Hering 2 ,
- Sonja C. Jähnig ORCID: orcid.org/0000-0002-6349-9561 8 , 10 ,
- Astrid Schmidt-Kloiber ORCID: orcid.org/0000-0001-8839-5913 11 ,
- Rachel Stubbington 12 ,
- Florian Altermatt ORCID: orcid.org/0000-0002-4831-6958 13 , 14 ,
- Mario Álvarez-Cabria ORCID: orcid.org/0000-0003-2709-5030 15 ,
- Giuseppe Amatulli 16 ,
- David G. Angeler ORCID: orcid.org/0000-0003-2197-7470 17 , 18 , 19 , 20 ,
- Gaït Archambaud-Suard 21 ,
- Iñaki Arrate Jorrín 22 ,
- Thomas Aspin 23 ,
- Iker Azpiroz 24 ,
- Iñaki Bañares 25 ,
- José Barquín Ortiz ORCID: orcid.org/0000-0003-1897-2636 15 ,
- Christian L. Bodin 26 ,
- Luca Bonacina ORCID: orcid.org/0000-0002-4695-5932 27 ,
- Roberta Bottarin ORCID: orcid.org/0000-0002-6352-3699 28 ,
- Miguel Cañedo-Argüelles ORCID: orcid.org/0000-0003-3864-7451 7 , 29 ,
- Zoltán Csabai ORCID: orcid.org/0000-0003-1700-2574 30 , 31 ,
- Thibault Datry 32 ,
- Elvira de Eyto ORCID: orcid.org/0000-0003-2281-2491 33 ,
- Alain Dohet ORCID: orcid.org/0000-0003-2962-7387 34 ,
- Gerald Dörflinger ORCID: orcid.org/0000-0003-0209-4648 35 ,
- Emma Drohan ORCID: orcid.org/0000-0002-3391-3100 36 ,
- Knut A. Eikland ORCID: orcid.org/0000-0001-5037-7509 37 ,
- Judy England ORCID: orcid.org/0000-0001-5247-4812 38 ,
- Tor E. Eriksen ORCID: orcid.org/0000-0003-2033-6399 39 ,
- Vesela Evtimova ORCID: orcid.org/0000-0002-6358-8011 40 ,
- Maria J. Feio ORCID: orcid.org/0000-0003-0362-6802 41 ,
- Martial Ferréol ORCID: orcid.org/0000-0001-6740-3654 32 ,
- Mathieu Floury ORCID: orcid.org/0000-0002-4952-5807 8 , 42 ,
- Maxence Forcellini ORCID: orcid.org/0000-0003-4921-2189 32 ,
- Marie Anne Eurie Forio 43 ,
- Riccardo Fornaroli ORCID: orcid.org/0000-0001-6326-5653 27 ,
- Nikolai Friberg 39 , 44 , 45 ,
- Jean-François Fruget 46 ,
- Galia Georgieva ORCID: orcid.org/0000-0003-3367-3802 40 ,
- Peter Goethals 43 ,
- Manuel A. S. Graça ORCID: orcid.org/0000-0002-6470-8919 41 ,
- Wolfram Graf 11 ,
- Andy House 23 ,
- Kaisa-Leena Huttunen ORCID: orcid.org/0000-0003-0488-1274 47 ,
- Thomas C. Jensen ORCID: orcid.org/0000-0003-2777-2759 37 ,
- Richard K. Johnson 17 ,
- J. Iwan Jones ORCID: orcid.org/0000-0002-7238-2509 48 ,
- Jens Kiesel ORCID: orcid.org/0000-0002-4371-6434 8 , 49 ,
- Lenka Kuglerová 50 ,
- Aitor Larrañaga 51 ,
- Patrick Leitner 11 ,
- Lionel L’Hoste ORCID: orcid.org/0000-0003-0239-9468 34 ,
- Marie-Helène Lizée 21 ,
- Armin W. Lorenz ORCID: orcid.org/0000-0002-3262-6396 2 ,
- Anthony Maire ORCID: orcid.org/0000-0003-0920-773X 52 ,
- Jesús Alberto Manzanos Arnaiz 22 ,
- Brendan G. McKie 17 ,
- Andrés Millán 53 ,
- Don Monteith ORCID: orcid.org/0000-0003-3219-1772 54 ,
- Timo Muotka 47 ,
- John F. Murphy ORCID: orcid.org/0000-0003-2102-7686 48 ,
- Davis Ozolins ORCID: orcid.org/0000-0003-3752-2040 55 ,
- Riku Paavola ORCID: orcid.org/0000-0002-4708-1413 56 ,
- Petr Paril ORCID: orcid.org/0000-0002-7471-997X 31 ,
- Francisco J. Peñas 15 ,
- Francesca Pilotto ORCID: orcid.org/0000-0003-1848-3154 37 ,
- Marek Polášek ORCID: orcid.org/0000-0003-3213-7135 31 ,
- Jes Jessen Rasmussen ORCID: orcid.org/0000-0002-5932-3125 39 ,
- Manu Rubio ORCID: orcid.org/0000-0001-5225-9557 24 ,
- David Sánchez-Fernández ORCID: orcid.org/0000-0003-1766-0761 53 ,
- Leonard Sandin 37 ,
- Ralf B. Schäfer ORCID: orcid.org/0000-0003-3510-1701 57 ,
- Alberto Scotti 28 , 58 ,
- Longzhu Q. Shen 8 , 59 ,
- Agnija Skuja 55 ,
- Stefan Stoll ORCID: orcid.org/0000-0002-3656-417X 2 , 60 ,
- Michal Straka 31 , 61 ,
- Henn Timm 62 ,
- Violeta G. Tyufekchieva ORCID: orcid.org/0000-0001-9324-0430 40 ,
- Iakovos Tziortzis ORCID: orcid.org/0000-0002-9315-7773 35 ,
- Yordan Uzunov 40 ,
- Gea H. van der Lee ORCID: orcid.org/0000-0002-8731-682X 63 ,
- Rudy Vannevel 43 , 64 ,
- Emilia Varadinova ORCID: orcid.org/0000-0002-2382-9191 40 , 65 ,
- Gábor Várbíró ORCID: orcid.org/0000-0001-5907-3472 66 ,
- Gaute Velle ORCID: orcid.org/0000-0002-5603-271X 26 , 67 ,
- Piet F. M. Verdonschot ORCID: orcid.org/0000-0002-4126-7452 63 , 68 ,
- Ralf C. M. Verdonschot ORCID: orcid.org/0000-0002-0977-5975 63 ,
- Yanka Vidinova ORCID: orcid.org/0000-0002-6438-2349 40 ,
- Peter Wiberg-Larsen 69 &
- Ellen A. R. Welti ORCID: orcid.org/0000-0001-6944-3422 1 , 70 na1
Nature volume 620 , pages 582–588 ( 2023 ) Cite this article
35k Accesses
61 Citations
544 Altmetric
Metrics details
- Freshwater ecology
Owing to a long history of anthropogenic pressures, freshwater ecosystems are among the most vulnerable to biodiversity loss 1 . Mitigation measures, including wastewater treatment and hydromorphological restoration, have aimed to improve environmental quality and foster the recovery of freshwater biodiversity 2 . Here, using 1,816 time series of freshwater invertebrate communities collected across 22 European countries between 1968 and 2020, we quantified temporal trends in taxonomic and functional diversity and their responses to environmental pressures and gradients. We observed overall increases in taxon richness (0.73% per year), functional richness (2.4% per year) and abundance (1.17% per year). However, these increases primarily occurred before the 2010s, and have since plateaued. Freshwater communities downstream of dams, urban areas and cropland were less likely to experience recovery. Communities at sites with faster rates of warming had fewer gains in taxon richness, functional richness and abundance. Although biodiversity gains in the 1990s and 2000s probably reflect the effectiveness of water-quality improvements and restoration projects, the decelerating trajectory in the 2010s suggests that the current measures offer diminishing returns. Given new and persistent pressures on freshwater ecosystems, including emerging pollutants, climate change and the spread of invasive species, we call for additional mitigation to revive the recovery of freshwater biodiversity.
Similar content being viewed by others
Multi-decadal improvements in the ecological quality of European rivers are not consistently reflected in biodiversity metrics

Past and recent anthropogenic pressures drive rapid changes in riverine fish communities
Time series of freshwater macroinvertebrate abundances and site characteristics of European streams and rivers
Freshwater ecosystems are biodiversity hotspots and provide vital ecosystem services, including drinking water, food, energy and recreation. However, humans have degraded freshwaters for centuries, with impacts sharply increasing after World War II during the great acceleration 3 . Freshwaters are exposed to anthropogenic pressures from agricultural and urban land uses over whole catchments, accumulating pollutants, including phosphorus, organic-rich effluents, fine sediments, pesticides and emergent pollutants (such as nanoplastics and pharmaceuticals) 4 , 5 . Furthermore, freshwaters have been degraded by hydromorphological alterations, water extraction, invasive species and climate change 6 , 7 . In response to legislation such as the US Clean Water Act (1972) and the EU Water Framework Directive (2000), key countermeasures designed to improve water quality and restore freshwater habitats were implemented, including better wastewater treatment and controls on the emission of airborne pollutants. These actions resulted in considerable declines in organic pollution and acidification beginning around 1980 8 . Over the past 50 years, such mitigation measures have resulted in quantifiable improvements in freshwater biodiversity in some locations 9 , yet the number and impacts of stressors threatening freshwater ecosystems continues to increase worldwide and the biological quality of rivers remains poor globally 10 , 11 .
Freshwater invertebrates are a phylogenetically and ecologically diverse group that contribute to critical ecosystem processes, including decomposing organic matter, filtering water, providing energy to higher trophic levels, and transporting nutrients and energy between aquatic and terrestrial ecosystems 12 , 13 . Moreover, freshwater invertebrates have long been a cornerstone of water-quality monitoring. The biological traits of freshwater invertebrates are well characterized, enabling the assessment of functional diversity—the range of functional traits of the organisms in a given ecosystem 14 —an important facet of biodiversity that can be used as a proxy for ecosystem functioning 15 , 16 . However, trajectories of taxonomic and functional diversity have rarely been investigated simultaneously at larger spatial and temporal scales. Determining the trajectories of taxonomic and functional change could inform the development of evidence-based management strategies that address stressors through mitigation, restoration and conservation. Furthermore, how temporal changes in biodiversity manifest across large spatial scales and vary among taxonomic groups remains equivocal 17 , 18 , 19 . Examining whole ecological groups representative of a particular ecosystem (for example, freshwater invertebrate communities in river ecosystems) may help to clarify discrepancies across studies and identify key drivers of temporal change.
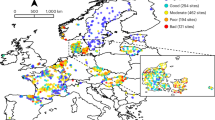
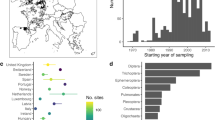
Here we analysed pan-European patterns and drivers of multidecadal trends in abundance and taxonomic and functional diversity of invertebrate communities using a comprehensive dataset of 1,816 time series collected in riverine systems in 22 European countries between 1968 and 2020 (Fig. 1 ). The dataset comprises 714,698 observations of 2,648 taxa in 26,668 samples. The time series span a mean of 19.2 years with an average of 14.9 sampling years (minimum 8 years, maximum 32 years). We address two research questions: (1) how abundance, taxonomic diversity and functional diversity of freshwater invertebrate communities have changed over the past five decades in European streams and rivers; and (2) what environmental factors have driven these changes. Given that Europe-wide management has resulted in improvements in water quality 2 , 20 , we hypothesize that abundance, taxonomic diversity and functional diversity have increased, consistent with a recovery. We further hypothesize that freshwater invertebrate community recovery was strongest around the end of the previous century after the onset of concerted efforts to mitigate stressor impacts and restore ecosystems, but has slowed in recent years owing to diminishing returns on these actions in addition to remaining and new pressures including climate change, land-use intensification and emerging pollutants. We assessed evidence for negative impacts of multiple human pressures, including dams, urban areas and cropland, and increasing temperatures, while accounting for subcatchment characteristics (such as elevation and stream size). We used hierarchical Bayesian models to estimate trends and identify drivers of change in abundance and taxonomic and functional diversity of Europe’s freshwater invertebrate communities, while accounting for temporal autocorrelation, sampling date and sampling variation across studies and countries.
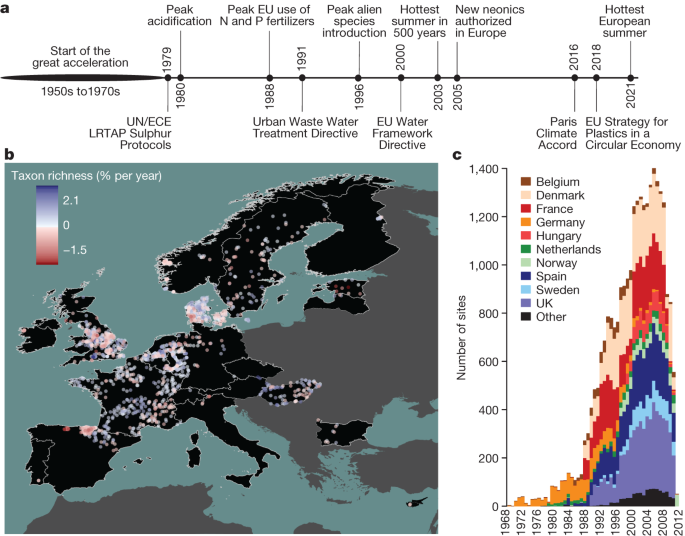
a , A timeline of major stressors (above the line) and environmental legislation (below the line) affecting Europe’s freshwater ecosystems (citations are provided in Supplementary Table 1 ). UN/ECE LTRAP, United Nations Economic Commission for Europe Long-Range Transboundary Air Pollution. b , The sampling sites (points) and the rate of temporal change in taxon richness of freshwater invertebrate communities (colour of points) across 22 European countries (black). c , The distribution of sampling sites over time and countries. ‘Other’ includes countries with fewer than 50 sampling sites.
Recovery of Europe’s freshwater invertebrate communities
Across all time series, taxon richness increased by 0.73% per year, whereas abundance increased by 1.17% per year between 1968 and 2020 (Fig. 2a,b ), substantiating previous documentation of a recovery process 18 , 21 , 22 . The probabilities of trends derived from posterior distributions (that is, the probability of the mean trend being above or below zero) revealed 0.99 and 0.91 probabilities of a mean increase in taxon richness and abundance, respectively. Despite these net-positive trends, taxon richness declined at 30% of sites and abundance declined at 39% of sites. Abundance trends for EPT taxa (mayflies, stoneflies and caddisflies—an indicator group of water quality 23 ) and insects increased (EPT, +2.38% per year, 0.97 probability; insects, +1.53% per year, 0.95 probability) at higher net rates than the overall trends. EPT richness (+0.45% per year, 0.82 probability) and insect richness (+0.71% per year, 0.99 probability) trends increased, but at net rates lower than the overall trends (Extended Data Fig. 1 ).
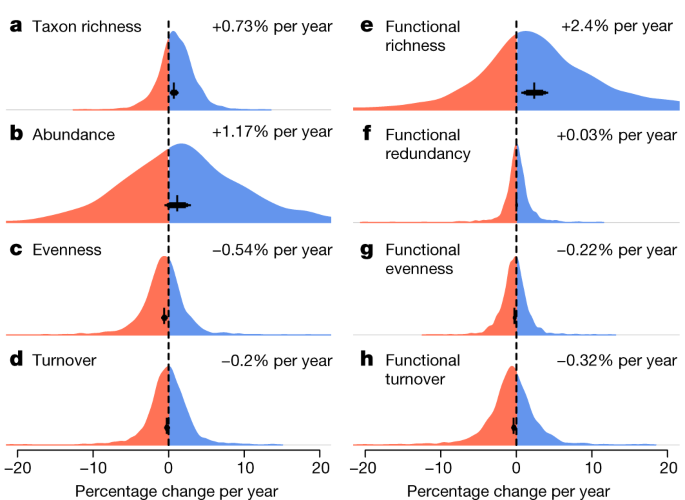
a – h , Overall meta-analysis estimates and distributions of site-level trends for taxonomic metrics of taxon richness ( a ), abundance ( b ), Shannon’s evenness ( c ) and turnover ( d ), and functional metrics of richness ( e ), redundancy ( f ), evenness ( g ) and turnover ( h ) across all 1,816 sites. The black error bars and text on each panel show the mean estimates (percentage change per year). The error bars indicate the 80%, 90% and 95% CIs.
Freshwater ecosystems are frequently invaded by non-native species 7 . We therefore examined whether changes in abundance and richness were driven by these taxa. Non-native species comprised an average of 4.9% of the species and 8.9% of the individuals at the 1,299 sites for which the taxonomic resolution allowed detection. Thus, native species dominated most communities (with 99.9% of sites comprising >50% native species). When considering only native taxa, trends in richness (+0.64% per year, 0.98 probability) and abundance (+0.26% per year, 0.61 probability) remained positive, but less so than overall net trends (Fig. 2 and Extended Data Fig. 1 ). For sites at which non-native species were detected (898 out of 1,299 sites), non-native species richness (+3.97% per year, 0.99 probability) and abundance (+3.9% per year, 0.95 probability) increased sharply (Extended Data Fig. 1 ).
Functional diversity, which describes the value and range of functional traits of the organisms in a given ecosystem 14 (Supplementary Table 4 ), also increased over the 53-year study period. Functional richness, which quantifies the functional space filled by a community, increased on average by 2.4% per year (0.99 probability of increase; Fig. 2e ). Functional redundancy—a measure of overlap in functional trait space—had no strong trend (+0.03% per year, 0.64 probability of increase; Fig. 2f ). By contrast, functional evenness declined (−0.22% per year, 0.96 probability of decrease; Fig. 2g ), as did taxonomic evenness (−0.54% per year, 0.99 probability; Fig. 2c ). Similarly, functional temporal turnover (−0.32% per year, 0.97 probability; Fig. 2h ) and taxonomic temporal turnover declined (−0.2% per year, 0.87 probability; Fig. 2d ). Together, these results suggest that functional diversity trends largely paralleled those of taxonomic diversity. Model estimates and raw distributions of trends for additional taxonomic and functional metrics are shown in Extended Data Fig. 2 .
Gains in species richness have come to a halt
While overall net trends provide an overview across the entire study period and enable comparison with other long-term biodiversity studies 17 , 19 , 24 , they may mask important shorter-term temporal fluctuations in trends. Thus, to provide more nuanced, complementary trend information, we used a ten-year moving-window approach to examine the trajectories of freshwater invertebrate community change over time. Nonlinear trajectories were expected due to temporal variation in pressures and the implementation of mitigation measures 25 . To improve spatial representativity and comparability across years, only years with at least 250 sites from at least 8 countries were included, corresponding to the period of 1990 to 2020.
Although trends in taxon richness were generally positive, indicating increases in local richness through time, this effect became weaker over the decades (mean change in trends = −8.8% per year, 95% credible interval (CI) range: −13.6% to −3.8% per year). Trends in taxon richness started declining around 2010 and then levelled off, reaching an average of net zero around 2013 (Fig. 3a ), indicating an end to the preceding recovery period. When considering only the dominant pattern as measured by the proportion of positive trends, the proportion of sites with increasing taxon richness declined after windows centred on the early 2000s (Fig. 3e ). Functional richness trends were more variable, with the highest trends evident for windows centred on 2000 and 2010, and near net zero trends after 2010 (Fig. 3c ). Functional richness trends had an overall tendency to decline (mean change in trends of functional richness = −5.9% per year, 95% CI range: −12% to +0.1% per year). Temporal changes in the proportion of sites with positive functional richness trends were similar to those reported for taxon richness (Fig. 3e,g ). Trends in abundance (Fig. 3b,f ) and functional redundancy (Fig. 3d,h ) changed little over time (that is, CIs overlapped with zero in an analysis of the change in trend estimates over time), although abundance trends tended to decline from windows centred on 2010 until the end of the study period.
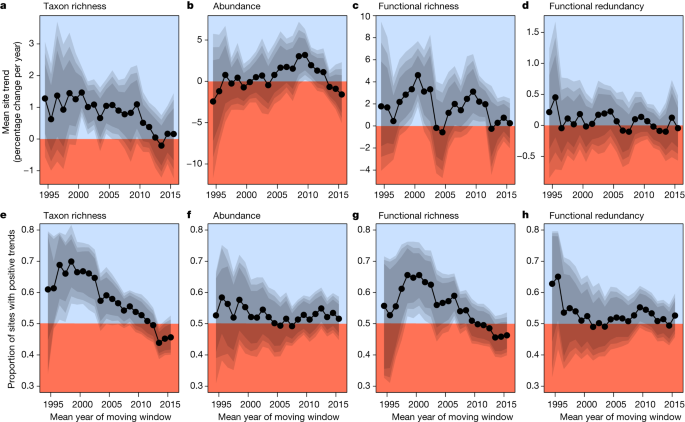
a – h , Modelled trend estimates from moving windows of taxon richness ( a ), abundance ( b ), functional richness ( c ) and functional redundancy ( d ), and the proportion of sites with positive trend estimates of taxon richness ( e ), abundance ( f ), functional richness ( g ) and functional redundancy ( h ). Trend estimates were calculated from Bayesian mixed-effects models of trends from at least 250 time series with at least 6 years of data from at least 8 countries within 10-year moving windows (totalling 21,495 time-series segments). The proportions are based on whether site-level trend estimates of these time-series were above zero or not. For trend estimates in a – d , blue and red areas indicate the overall positive (>0) and negative (<0) mean trend estimates for the given 10-year window, respectively, and the grey polygons indicate the 80%, 90% and 95% CIs. For site proportions in e – h , blue and red areas indicate a larger proportion of positive (>50% of sites) and negative (<50% of sites) site-level trend estimates for the given 10-year window, respectively, and the grey polygons indicate 80%, 90% and 95% CIs.
Although similar trends in taxonomic and functional metrics were expected due to functional variation being constrained by taxon richness, functional diversity can be more responsive to environmental gradients 26 . However, changes in functional diversity have rarely been quantified in large-scale investigations of temporal change in biodiversity 27 , 28 . A switch from primarily positive trends in functional richness in the late 1990s and early 2000s to near-zero trends starting around 2012 (Fig. 3c ) may suggest no further improvements in ecosystem functioning. The concurrent limited change in functional redundancy (Fig. 3d ) indicates that the increase in functional richness provided new traits to these communities rather than adding traits that were already present. Both taxonomic and functional trends in evenness and turnover remained near zero or slightly negative over time (Extended Data Fig. 3 ).
Environmental drivers of biodiversity change
Identifying the natural and anthropogenic drivers of biotic change is critical to inform effective management strategies. Here we show that climate, dam impacts, and the percentage of upstream urban areas and cropland (both sources of pollution and causes of habitat degradation) can all be linked to trends in taxonomic and functional metrics representing Europe’s freshwater invertebrate communities (Fig. 4 and Extended Data Figs. 4 and 5 ).
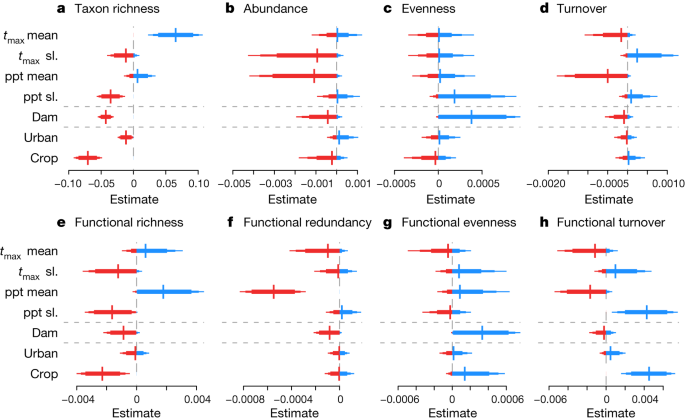
a – h , Estimated effects of the mean ( t max mean) and trend ( t max slope (sl.)) of annual maximum monthly mean temperatures, mean (ppt mean) and trend (ppt sl.) of the annual cumulative precipitation, the dam impact score (dam) and the percentage of the upstream catchment covered by urban areas and cropland on site-level long-term trend estimates for taxon richness ( a ), abundance ( b ), evenness ( c ) and turnover ( d ), and functional richness ( e ), redundancy ( f ), evenness ( g ) and turnover ( h ). n = 1,816 biologically independent sites for all metrics. Positive and negative estimates are shown in blue and red, respectively. For climatic drivers, mean values refer to mean long-term values at each site and represent geographical variation; trends were calculated by regressing annual mean values against year, using the coefficient as an estimate of climatic trend and represent temporal variation. All response variables are site-level trends (that is, change in biodiversity metric over time) and all covariates were standardized to units of s.d. before analysis. A positive coefficient means that sites with higher values of the driver tended to have higher trends, although not necessarily positive trends, compared with sites with lower values of the driver. For example, trends in taxon richness were higher at sites with higher maximum mean temperatures ( t max mean) but lower at sites with higher rates of temperature increase ( t max sl.; b ). The bars around the estimates indicate 80%, 90% and 95% CIs. The grey horizontal lines separate the three environmental driver groups: climate, dams and land use. Estimates of stream characteristics (stream order, flow accumulation, elevation and slope) are shown in Extended Data Fig. 6 .
Climate strongly influenced freshwater invertebrate communities (Fig. 4 ). Overall, sites experienced a net increase in air temperature of +0.037 °C per year ± 0.0007 s.e.m. (with 94% of sites warming) and a slight net increase in precipitation of +0.49 mm per year ± 0.12 s.e.m. (with 57% of sites getting wetter) over the studied intervals. Sites in areas with higher mean air temperatures were more likely to gain taxa (Fig. 4 ) compared with those in cooler areas. This may indicate that climate warming has not yet reached critical values for many European freshwater invertebrates, consistent with previous predictions for ectotherms in temperate regions 29 , 30 . Alternatively, lower recovery rates for biotic communities in cooler areas could reflect the less severe degradation of northern sites before recovery started. By contrast, more warming over time had negative biodiversity outcomes, with negative effects on long-term trends of taxon richness, abundance and functional richness (Fig. 4 ). Mean precipitation had a positive effect on long-term trends of functional richness but a negative effect on long-term trends of abundance and functional redundancy, indicating the addition of functionally unique taxa at wet sites. However, greater increases in precipitation over time had a negative effect on long-term trends of both taxonomic and functional richness (Fig. 4 ). Precipitation can influence invertebrate communities and their functioning by altering flow regimes (and therefore water quality and temperature through changes in runoff, discharge and dilution) and food availability 6 .
Biodiversity trends were generally lower at sites downstream of dams and in catchments with a high percentage of urban areas or cropland. High dam impacts (that is, those in systems connected to more dams and/or closer to dams) had negative effects on long-term trends in taxon richness, abundance, functional richness and functional redundancy (Fig. 4 ). Dams increase sediment loads, reduce longitudinal connectivity, and change river flow and temperature regimes 31 , 32 , 33 . By contrast, high dam impacts had a positive effect on long-term trends of both taxonomic and functional evenness, suggesting that dominant species declined in abundance in communities downstream of dams, whereas richness losses were more pronounced for rare species. Furthermore, increases in functional evenness, accompanied by decreases in functional richness and redundancy, could reflect selection for a subset of traits that confer tolerance of the conditions downstream of dams, including altered resource availability and hydromorphological homogenization. A greater percentage of upstream cropland had a negative effect on long-term trends in taxonomic and functional richness and abundance. Cropland frequently contributes to nutrient-enriched runoff, leaving primarily tolerant taxa 34 . A greater percentage of upstream urban areas had negative effects on taxon richness long-term trends (Fig. 4 ), but positive effects on non-native richness long-term trends (Extended Data Fig. 5a ), suggesting losses of rare and sensitive native species. Biodiversity trends varied little with stream characteristics, although sites at higher elevations had lower gains in functional richness, potentially due to rising temperatures (as evidenced by a weak positive correlation between temperature trends and elevation; r = 0.15) 35 . Larger rivers became relatively more prone to invasion by non-native species 36 (Extended Data Figs. 6 – 8 ).
Reviving the recovery
Using a comprehensive Europe-wide dataset, we document the recovery of freshwater invertebrate communities over the past 53 years. The taxon richness gains observed across 70% (1,269 out of 1,816) of time series are concurrent with widespread implementation of mitigation measures 8 , particularly improvements in wastewater treatment motivated by the EU Urban Waste Water Directive from 1991. However, gains in taxon richness started to decelerate around 2010, which may indicate that progress towards recovery has come to a halt at many sites, while remaining sites may reflect either predominant recovery or ongoing degradation towards the end of the study period. Most of our sites are monitored under the EU Water Framework Directive (WFD) and 60% of WFD-monitored rivers still do not reach ‘good ecological status’ 37 . Even at ‘good’ sites, considerable recovery could be needed to reach ‘high ecological status’, suggesting that improvements documented here represent only a partial recovery of European freshwater ecosystems.
Regardless of the reason for the deceleration, the impacts to Europe’s rivers caused by ongoing pressures remain extensive and severe 37 , 38 . Although our observational data prevent confirmation of the underlying causal processes, our interpretation of the overall recovery being a response to improving water quality aligns with the conclusions of other studies of European freshwater invertebrate time series 9 , 39 . Negative effects of poor water quality on biodiversity are supported by our findings that freshwater invertebrate communities downstream of dams, urban areas and cropland were less likely to experience biodiversity recovery. Urban areas produce the majority of micropollutants, are hubs of non-native species invasions (Extended Data Fig. 5a ) and generate high-nutrient inputs, whereas croplands are sources of fine sediment 40 , pesticides and nutrient-laden runoff 41 , and greatly contribute to river salinization 42 . Most European rivers bear a substantial legacy of human impacts on their hydromorphology 8 , 38 , with urban areas being the most affected, despite considerable river restoration in recent decades 43 . The positive effects of higher mean temperatures on long-term trends in invertebrate richness probably reflect the lower initial degradation in northern European countries. This may also reflect the relatively cool temperatures in European countries, whereas decreases in invertebrate richness are currently expected in freshwaters of warmer bioregions, such as tropical regions, which are not represented in our study 44 . However, the negative effects on long-term trends of taxon richness, abundance and functional richness in communities experiencing greater rates of warming are worrying. These effects are likely to worsen as temperatures continue to rise and as climatic extremes including summer droughts and heatwaves become more common 45 .
Considering that environmental legislation and policy have insufficiently addressed ongoing and emerging stressors 8 , the stalled recovery is unsurprising. Further management actions to revive the recovery should target sites at greater risk of biodiversity decline, such as those downstream of urban areas, cropland and dams, while maintaining and strengthening protection of the least impacted systems that are refuges of biodiversity. Specifically, substantial, catchment-scale changes in land management must go beyond current legislative requirements and achieve greater reductions in water extraction and inputs of pollutants including fine sediments, pesticides and fertilizers. Substantial investment is needed to upgrade sewage networks and improve wastewater treatment plants to better manage stormwater overflow and more effectively remove micropollutants, nutrients, salts and other contaminants 46 . Adopting a catchment-scale approach that considers barriers to dispersal 47 can further enhance the effectiveness of management, conservation and restoration practices 32 , 48 . Additional hydromorphological restoration efforts are required to reconnect rivers and floodplains to improve ecosystem functioning, prevent destructive floods, and adapt riverine systems to future climatic and hydrological regimes. Finally, standardized, large-scale and long-term biodiversity monitoring, paired with parallel environmental data collection 49 , 50 , should be prioritized to effectively characterize temporal changes in biodiversity and environmental drivers and identify sites at high risk 51 .
Current large-scale measures to address biodiversity loss remain rare, especially for invertebrates. This in part reflects our understanding of biodiversity change, which is limited by unknown historical baseline conditions and complex variation in interacting anthropogenic stressors. Insufficient baseline data present challenges both for characterization of biodiversity trends and ecological status of communities, and evaluation of tolerable levels and effects of stressors 52 . Data on the state of freshwater communities both before and during the great acceleration are largely lacking, making it unclear when freshwater degradation peaked. Long-term data from the UK suggest freshwater invertebrate biodiversity was lowest at the start of the 1990s 53 , but our pre-1990s data are insufficient to determine whether this pattern is Europe-wide (Fig. 1c ). Moreover, comparison with unimpacted ‘reference’ communities, a standard practice in freshwater ecology, is becoming increasingly challenging due to the emergence of new communities 54 resulting from climate change, non-native species invasions and other pressures 55 . Progress towards biodiversity goals needs to recognize these changing pressures through flexible strategies to protect and foster Earth’s remaining biodiversity. We call for adaptive environmental management that recognizes conservation and restoration objectives as shifting targets that can be modified to adapt to global change and maximize the protection of biodiversity.
Time series
We assembled a database of time series of riverine invertebrate communities following a data call targeting European ecologists and environmental managers. We included only time series that (1) included abundance estimates; (2) documented whole freshwater invertebrate communities (including all sampled macroinvertebrates, for example, Coleoptera, Crustacea, Diptera, Ephemeroptera, Hirudinea, Mollusca, Odonata, Oligochaeta, Plecoptera, Trichoptera, Tricladida); (3) identified most taxa to family, genus or species; (4) had ≥8 sampling years (not necessarily consecutive); (5) used the same sampling method and taxonomic resolution throughout the sampling period; and (6) had consistent sampling effort per site (for example, the number of samples or area sampled) in all years.
Only one sampling event per year was included for each time series, where a sampling event was defined as the sample or samples collected within a single day. For time series with multiple sampling seasons within or among years, we included only one sampling season (defined as three consecutive months), preferentially using the season with the longest time series. No time series had multiple sampling events per season. Sensitivity analyses indicated limited effects of season on trend estimates (Extended Data Fig. 10 ). We removed taxa that are not freshwater invertebrates, including terrestrial and semi-aquatic taxa, and vertebrates, in addition to freshwater invertebrates that were recorded inconsistently owing to their small size (such as mites, copepods and cladocerans).
Between 13 and 516 taxa were sampled per site across all sampling years. Communities from 42% of sites were identified to species, 30% were identified to mixed (species-to-family) taxonomic levels and 28% were identified primarily to family. In total, 2,648 taxa from 959 genera, 212 families and 47 groups (primarily orders) were recorded. We list time-series locations, durations and characteristics in Supplementary Table 2 and list the number of sites sampled per year and country in Supplementary Table 3 .
Our compiled time series represent different stream types and stream orders from a large geographical area of Europe. Data were collected for purposes including research projects and regulatory biomonitoring, although detailed information on the purpose is unavailable for some time series. These data were not selected randomly but were collected from available studies that met our six criteria. As these data were collected from sites exposed to varying and unquantified levels of anthropogenic impacts, we cannot rule out biases arising from unequal representation of sites exposed to different impact levels from severely impacted to least impacted.
Community metrics
We calculated taxonomic and functional diversity metrics representing freshwater invertebrate communities across sites and over time. We also examined different community subsets: native and non-native species, and insects and EPT taxa (Ephemeroptera, Plecoptera, Trichoptera, that is, mayflies, stoneflies, caddisflies, grouped as an indicator of water quality 56 ).
Taxonomic diversity
We calculated total abundance, taxon richness, Shannon’s diversity, Shannon’s evenness, rarefied richness (calculated on the basis of standardized numbers of individuals) and temporal turnover for each site and year. As sampling effort was standardized within time series before metric calculation, individual-based rarefied richness was used to estimate the number of taxa per given number of individuals, based on the lowest number of individuals per sampling year in each time series 17 . We calculated temporal turnover as the ratio of taxa gained or lost to the total number of taxa present between two timepoints using the R package codyn 57 . All other taxonomic metrics were calculated using the R package vegan 58 .
Functional diversity
Traits were extracted from the European databases freshwaterecology.info (v.7.0) 59 and DISPERSE 60 . First, we downloaded trait data for all taxa. We considered biological traits that influence both a taxon’s response to and its effects on its environment 61 , 62 . Specifically, we compiled data on 10 biological traits (with 53 trait modalities): respiration type, resistance form, dispersal type, aquatic stage, life cycle duration, reproduction type, maximum potential body size, wing form, propensity to drift and feeding type 60 , 63 . For taxa with multiple aquatic life stages (primarily beetles), whenever available from the trait databases, functional roles were assigned for each life stage, otherwise adult traits were used. We included only traits for which information was available for >85% of all taxa. All traits were fuzzy coded across multiple modalities depending on the information available; for example, the trait ‘maximum potential body size’ contains seven modalities ranging from ≤0.25 cm to >8 cm. Within each trait, we scaled affinities to different component modalities between 0 and 1 (summing to 1 across modalities for each taxon), so that each taxon was assigned an affinity score for each modality 64 , to recognize potential trait plasticity.
We took the following steps to fill in gaps due to missing trait data. First, when trait data were not available at the original identification level (15.9% trait coverage across taxa), we used genus-level trait data, resulting in 48.2% coverage. Genus-level trait data are generally sufficient to represent most interspecific variation among freshwater invertebrates and thus taxon responses to environmental variability 61 . Next, when genus-level trait data were not available for taxa identified to genus, we replaced missing values in trait modalities with the median of trait profiles of all species within a genus from the full taxon list, resulting in 61.3% coverage. For taxa identified to family level with no available data for a given trait, we replaced missing values in trait modalities with the median value of trait profiles of all genera within a family, resulting in 90.5% coverage across all taxa. The lack of accurate phylogenies for many invertebrate taxa, low trait coverage at the species level and mixed taxonomic resolution across sampling sites prevented the use of other gap-filling approaches, but taxonomic aggregation generally aligns well with expert trait assignments 65 .
We analysed functional diversity separately for each site by calculating six distance-based metrics chosen to describe multiple facets of community niche space and to align with taxonomic diversity metrics: functional richness, functional redundancy, functional evenness, functional turnover, functional divergence and Rao’s quadratic entropy (definitions and citations are provided in Supplementary Table 4 ). All functional metrics except for functional redundancy and turnover were calculated using the dbFD function in the R package FD 66 . In calculations of functional richness and divergence, we used six principal coordinate analysis axes (the dbFD ‘m’ argument), according to current recommendations 67 . To enable calculation of functional turnover, we calculated community-weighted means of each functional trait category weighted by taxa abundance, then calculated turnover of the community-weighted means using the R package codyn, as for taxonomic temporal turnover 57 , 68 . We calculated abundance-weighted functional redundancy using the uniqueness function in the R package adiv 69 . We calculated redundancy according to a previous report 70 : community uniqueness ( U ) was calculated as quadratic diversity divided by Simpson diversity and functional redundancy was calculated as 1 − U . The trait input matrix was based on Euclidean distances bound between 0 and 1 and the tolerance threshold was 10 −8 .
Non-native species
Non-native species were defined as introduced species (that is, those present due to human activities, not natural range expansion) at the country level (for example, a species native to Bulgaria could be non-native in the UK). To identify non-native species, we used two databases: DAISIE 71 and the Global Alien First Record Database (GAFRD) (v.2) 72 , 73 . DAISIE contains non-native species in addition to native species defined as invasive because they cause economic loss (that is, pest species). GAFRD includes only non-native species but is limited to species and countries for which the approximate year of introduction is known. From each database, we first extracted all species listed for each European country in our dataset. We determined each species’ country of origin using the Global Biodiversity Information Facility 74 or peer-reviewed publications, both to eliminate native species listed in DAISIE and to check whether species listed as non-native in one European country were also non-native elsewhere (for example, a North American species marked as non-native in Germany in GAFRD would be non-native in all European countries in which it occurred).
In total, we identified 61 non-native species. The initial analysis of native and non-native species was restricted to the 1,299 sites at which taxa were identified to species or a mixed taxonomic resolution; we excluded the remaining 517 sites due to the coarse (primarily family level) taxonomic resolution, which does not allow for reliable identification of non-native species. Estimates of trends in non-native species richness and abundance were restricted to the 898 (of 1,299) sites at which non-native species were recorded. The two most abundant non-native species were the New Zealand mud snail, Potamopyrgus antipodarum (≥1 individual present in ≥1 year at 81% of sites) and the North American bladder snail, Physella acuta (34% of sites).
Stream characteristics and environmental predictors
Stream network.
We used the MERIT Hydro 75 digital elevation model (DEM) to delineate the high-resolution Hydrography90m stream network 76 . To achieve a high spatial accuracy, we used an upstream contributing area of 0.05 km 2 as the stream channel initialization threshold using the r.watershed and r.stream.extract modules in GRASS GIS 77 . We next calculated the subcatchments for each segment of the stream network, that is, the area contributing laterally to a given stream reach between two nodes, using the r.basins module. Coordinates indicating a site’s location did not always occur in the delineated stream network due to spatial inaccuracy of either the DEM or the coordinates. To ensure that point occurrences matched the DEM-derived stream network and therefore the network topology, we first identified the subcatchment in which each point occurrence was located, then moved all points to the corresponding stream segments using the v.net module within the given subcatchment. From each point, we calculated the network (as the fish swims) distance (km) using the v.net.distance module, and the Euclidean (as the crow flies) distance to all other point occurrences using the v.distance module. The distance was set to NA when sites were located in different drainage basins, and therefore not connected through the network.
Environmental predictors
We calculated stream topographical and topological predictors using the MERIT Hydro DEM 76 . Using the r.univar module in GRASS GIS, we computed the average elevation (m), elevation difference between the site and the upstream subcatchment (m), slope and the upstream contributing area (or flow accumulation, km 2 ) for each subcatchment. To create a proxy for dam impacts, we calculated the network distance between each site and each upstream dam using the Global Reservoir and Dam Database (v.1.3) 78 . For dam impact score calculations, see Supplementary equation ( 1 ).
We extracted monthly climatic predictors from the TerraClimate dataset 79 for 1967–2020, which covered all sites and years. For each site, we identified the sampling month and computed the mean monthly climatic value for the corresponding subcatchment. We calculated climatic predictors of cumulative annual precipitation (mm) and maximum monthly temperature (°C) for each 12 month period preceding the mean sampling month at each site. Trend values in precipitation and maximum temperature over the period covered by each time series were calculated using Bayesian models fitted using the R package brms 80 . These models were similar to those used to calculate site-level biodiversity metric trends, in which a trend was estimated as the coefficient of a continuous year effect. The TerraClimate dataset is associated with uncertainties in areas of complex terrain, but our large number of sampling sites, relatively good station coverage and the low physiographical complexity of most site locations should have minimized error in our analyses.
We calculated the proportion of land cover categories in each subcatchment using the ESA CCI Land Cover time series 81 for each year from 1992 to 2018. Land cover data were available for 92% of analysed site and year combinations and for 99% of sites. We computed the entire upstream catchment for each point occurrence using the r.water.outlet module and calculated the percentage cover of each land cover category within this area. The areas of cropland and urban land were calculated as the percentage of the upstream area averaged across the sampled years at each site.
A list of the stream characteristics and environmental drivers, their units and sources is provided in Supplementary Table 5 .
Statistical analysis
Trend analysis.
Temporal trends in each taxonomic (abundance, richness, Shannon’s diversity, Shannon’s evenness, individual-based rarefied richness and temporal turnover), functional (redundancy, richness, evenness, turnover, divergence and Rao’s quadratic entropy) and community subset (taxon richness and abundance of native species, non-native species, EPT taxa and insects only) metric were assessed using a two-step approach. First, we calculated site-level trends for each metric using Bayesian linear models fitted using the R package brms 80 . In these models, a biodiversity metric was the response variable and year was the continuous predictor variable of which the coefficient represented the temporal trend estimate.
The form of the model was: bf(BiodiversityMetric ~ cYear + ar(time = iYear, p = 1, cov = TRUE)).
Fixed-year variables were centred to improve model convergence (cYear) and year in the temporal autocorrelation term was included as a count with the first year of sampling considered year 1 (iYear). The models accounted for any residual temporal autocorrelation using an ar(1) term 82 and included day of year as an additional predictor when variation in sampling dates at a site was >30 days.
The form of the model was: bf(BiodiversityMetric ~ cday_of_year + cYear + ar(time = iYear, p = 1, cov = TRUE)).
The models assumed normally distributed errors, which were checked visually using histograms. Taxonomic evenness, functional richness, total abundance and subset abundance (non-native, native, EPT and insect abundance) were log 10 -transformed, and functional divergence was squared to meet the normality assumption.
We ran linear mixed-effects models (LMM) in the brms package to synthesize site-level data and estimate overall mean trends. The LMM included site-level trend estimates as the response, and an overall intercept and two random effects (country and study identity) as predictors. These random effects accounted for data heterogeneity due to unequal numbers of sites among studies and countries. Site-level trends were normally distributed; we therefore assumed normal errors. Site-level trends were combined in a meta-analysis model to estimate the mean trend across studies, including the uncertainty (represented by the s.d.) of the trend estimates, using brms 80 .
The form of the model was: brm(estimate|se(sd_trend_estimate) ~ 1 + (1|study_id) + (1|country), data = response_stan, iter = 5000, inits = 0, chains = 4, prior = c(set_prior(“normal(0,3)”, class = “Intercept”)), control = list(adapt_delta = 0.90, max_treedepth = 12)).
For each response metric, we calculated the proportion of the posterior distribution of the mean trend estimate (that is, the overall LMM intercept) above or below zero, that is, the probability of an increasing or decreasing mean trend.
In Bayesian models, we mostly used default brms settings, including four chains, which were run for 5,000 iterations (50% burn-in). We used default priors except for trend estimates, for which we selected a narrower prior to diminish the influence of biologically unrealistic trend estimates. Specifically, we used normally distributed priors with a mean of zero and an s.d. of 10 (for site-level trends) or 3 (for mean site-level trends). We compared our meta-analysis model of trends with and without including the uncertainty of site-level trend estimates. To optimize model fit, unweighted models were used for non-native and EPT abundance, and for EPT taxon richness. Functional turnover was fitted using beta models as values were bound between 0 and 1. The percentage change per year was calculated by back-transforming model estimates. Back-transformation calculations varied according to the originally modelled transformations of response variables (see the ‘equationsToPercChangePerYr.xlsx’ file in the ‘plots/Fig2_DensityPlots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ). We further tested a one-stage synthesis approach in which mean trends were estimated in one large mixed-effect model of the observed data, including random intercepts and slopes. Overall, these models produced similar trend results (see figure 16 in the ‘Online Figures.docx’ file in the ‘plots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ).
Moving-window analysis
To assess how estimates of trends in abundance and taxonomic and functional diversity changed over time, we used a moving-window approach. We used a similar two-stage process as described above. For each year of the analysis, we calculated trends within a ten-year window in which all time series with ≥6 sampling years and from ≥8 countries were included. A ten-year window was chosen according to current recommendations regarding times-series length 83 , 84 and six was chosen as the number of sampling years covering >50% of each ten-year period. This analysis was restricted to the period between a first moving window from 1990 to 1999, in which any time series with ≥6 sampling years was included, to a final window from 2011 to 2020. After estimating site-level trends centred on each year of the moving window, we ran a Bayesian LMM for each year to estimate the overall mean trends across sites in that time period. These models followed the same form as used to calculate trend estimates, containing the predictor variables of trends including an error term to account for uncertainty, an overall intercept, and study identity and country as random effects (see the equation in the ‘Trend analysis’ section).
To test for an overall linear change in the trajectory of moving-window trends, we modelled the effect of year on moving-window trend estimates using brms 80 .
The form of the model was: brm(MovingWindowTrend|se(sd_trend_estimate) ~ year, data = moving_window_trends, iter = 5000, inits = 0, chains = 4, prior = c(set_prior(“normal(0,3)”, class = “Intercept”)), control = list(adapt_delta = 0.90, max_treedepth = 12)).
These models identified a linear decline in trends in taxon richness and a tendency for decline in functional richness trends over time (see figure 21 in the ‘Online Figures.docx’ file in the ‘plots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ).
We examined the proportion of sites with positive trends and how this proportion changed through time for our key biodiversity metrics of taxon richness, abundance, functional richness and functional redundancy. To do this, we used site-level moving-window trends and estimated the proportion of sites with positive trends in each year. We repeated this calculation for each posterior draw to propagate through site-level uncertainty to the overall mean proportion and estimated 80%, 90% and 95% CIs. To ensure this proportion was not driven by studies with especially large numbers of sampling sites, we weighted each site by the inverse of the number of sites in each study. This complements the moving-window analysis by examining whether the emerging mean trends are typical of site-level patterns. This analysis was based only on trend direction and not trend magnitude and was therefore less affected by any noise contributed by studies with trends at the extremes.
An important caveat of the moving-window analysis is that different sites are included in different moving windows. Supplementary Table 6 lists the number of sites per window in each country. Although we accounted for the heterogeneity of site distribution across studies and countries within years, models cannot correct for the changing number of sampled sites across years. We cannot fully discount the possibility that biases in the characteristics of sites sampled across time affected trajectory results. We therefore conducted two additional moving-window analyses to investigate this, the first limited to sites with long-term data and the second limited to sites with species-level taxonomic resolution. The first additional analysis initially included only sites with ≥20 sampling years between 1990–2020, although moving windows with start years of 1990 and 1991 were excluded as they included <200 sites. This analysis included 308 sites from 8 countries. The second analysis included sites with species-level taxonomic data and windows covering 1990–2020 with >200 sites, resulting in windows from 1994–2003 to 2011–2020. The species-level moving-window analysis included 717 sites from 14 countries. Apart from the sites included, models were identical to our original moving-window analyses described above. These alternative moving-window analyses found similar declines in the trend of taxon richness over time (see figures 22–25 in the ‘Online Figures.docx’ file in the ‘plots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ).
Analysis of environmental predictors
We assessed responses of biodiversity metrics to climate (both the mean and the trend over the time series’ durations) and upstream land cover (as the annual mean cover value during the sampling period), dam impact score and subcatchment characteristics (Supplementary Table 5 ). We did not include upstream land-use trends as most sites exhibited low variation: cropland cover changed by a mean of −0.002% per year ± 0.11 s.e.m., with no change detected at 634 sites; urban cover changed by 2.48% per year ± 0.14 s.e.m., but with no change detected at 803 sites. To examine relationships between environmental drivers and biodiversity trends, we modelled trend estimates using an LMM, incorporating trend errors as for the calculation of the overall trend, including all predictor variables as fixed effects, and study identity and country as random effects.
The form of the model was: brm(estimate|se(sd) ~ PrecipTrend + TempTrend + PrecipMean + TempMean + StreamOrder + Accumulation + Elevation + Slope + Urban + Crop + DamScore + (1|study_id) + (1|country), = response_stan, iter = 5000, chains = 4, prior = prior1, control = list(adapt_delta = 0.90, max_treedepth = 12)).
We ran models using the R package brms 80 . We standardized predictor variables to unit s.d. to facilitate comparison of their relative importance. We used regularizing horseshoe priors on environmental covariates that pull unimportant covariate effects towards zero to avoid overfitting. Our analysis of drivers focused on site-level variation in long-term trends, and not temporal variation in short-term trends examined in the moving-window analysis. Thus, our driver analysis cannot be used to understand recent changes in trends. To further examine whether biodiversity trends were positive or negative across the range of driver values, we used R package marginaleffects 85 to visualize responses to drivers while holding other driver covariates at their median. Predicted trends complement the effects on trends shown in Fig. 4 (see figures 28–34 in the ‘Online Figures.docx’ file in the ‘plots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ).
Model checking
All models run to quantify biodiversity trends and responses to drivers were evaluated by plotting the posterior samples to confirm chain convergence, examining R -hat values (<1.1) 86 and estimating Pareto shape parameters using the argument pareto_k_table in the R package loo 87 . For trend models and across the 20 examined biodiversity metrics, an average of 99.5% of the 1,816 sites had shape parameter estimates of k < 0.7 (a threshold for good model performance). For environmental driver models, an average of 99% of the 1,816 sites had shape parameter estimates of k < 0.7.
Sensitivity analysis
To check the robustness of our results to analytical decisions, we ran multiple sensitivity analyses for all biodiversity metrics. We tested the effects on trend estimates of (1) taxonomic resolution, by rerunning meta-analysis models with resolution (family, mixed, and species) as an additional fixed factor; (2) sampling season, by rerunning meta-analysis models (described in the ‘Trend analysis’ section) with season (winter, spring, summer and fall) as an additional fixed factor; and (3) country, using a jackknife resampling analysis in which the meta-analysis was rerun after sequentially removing countries. Models were otherwise similar to those presented above. Scripts for sensitivity analyses are available at GitHub ( https://github.com/Ewelti/EuroAquaticMacroInverts (HPC_Sensitivity_analysis.R and HPC_Meta_analysis_country_jackknife).
Some caution is advised when inferring conclusions from a dataset including different levels of taxonomic resolution or different seasons. However, intra-site sampling was consistently within one season or taxonomic resolution, so intra-site trends were not affected by these differences. Neither taxonomic resolution nor season had strong directional effects on trend estimates, with error bars generally overlapping. Patterns across taxonomic resolutions and sampling seasons were generally similar to those presented in Fig. 2 (Extended Data Figs. 9 and 10 ). Trends of taxonomic richness were robust to one-country removal but abundance trends became more strongly positive on removal of data from some countries, suggesting geographical variability in abundance trends (see figure 17 in the ‘Online Figures.docx’ file in the ‘plots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ).
We analysed the effect of the number of sampling years in a time series on observed trends using simple linear regression. The number of sampling years did not affect trend estimates of taxon richness ( R 2 < 0.001), abundance ( R 2 < 0.001), functional richness ( R 2 = 0.004) or functional redundancy ( R 2 < 0.001) (see figure 14 in the ‘Online Figures.docx’ file in the ‘plots’ folder at https://github.com/Ewelti/EuroAquaticMacroInverts ).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to reproduce analyses including metadata, site characteristics and values of each metric (for example, species richness, functional richness) for each site and year are available at Figshare ( https://doi.org/10.6084/m9.figshare.22227841 ). Biodiversity composition data are available at GitHub ( https://github.com/Ewelti/EuroAquaticMacroInverts/raw-data ).
Code availability
Annotated R code is available at GitHub ( https://github.com/Ewelti/EuroAquaticMacroInverts ).
Dudgeon, D. et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81 , 163–182 (2006).
Article PubMed Google Scholar
Vaughan, I. P. & Ormerod, S. J. Large-scale, long-term trends in British river macroinvertebrates. Glob. Change Biol. 18 , 2184–2194 (2012).
Article ADS Google Scholar
Steffen, W., Broadgate, W., Deutsch, L., Gaffney, O. & Ludwig, C. The trajectory of the Anthropocene: the great acceleration. Anthr. Rev. 2 , 81–98 (2015).
Google Scholar
Windsor, F. M., Tilley, R. M., Tyler, C. R. & Ormerod, S. J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 646 , 68–74 (2019).
Article ADS CAS PubMed Google Scholar
Reid, A. J. et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94 , 849–873 (2019).
Mantyka-Pringle, C. S., Martin, T. G., Moffatt, D. B., Linke, S. & Rhodes, J. R. Understanding and predicting the combined effects of climate change and land-use change on freshwater macroinvertebrates and fish. J. Appl. Ecol. 51 , 572–581 (2014).
Article Google Scholar
Seebens, H. et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8 , 14435 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar
European Environment Agency (EEA). European Waters: Assessment of Status and Pressures 2018 EEA report 7/2018, https://www.eea.europa.eu/publications/state-of-water (2018).
Vaughan, I. P. & Gotelli, N. J. Water quality improvements offset the climatic debt for stream macroinvertebrates over twenty years. Nat. Commun. 10 , 1956 (2019).
Article ADS PubMed PubMed Central Google Scholar
Schwarzbach, S. E., Albertson, J. D. & Thomas, C. M. Effects of predation, flooding, and contamination on reproductive success of California clapper rails ( Rallus longirostris obsoletus ) in San Francisco Bay. Auk 123 , 45–60 (2006).
Birk, S. et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 4 , 1060–1068 (2020).
Vaughn, C. C. & Hakenkamp, C. C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 46 , 1431–1446 (2001).
Vanni, M. J. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Evol. Syst. 33 , 341–370 (2002).
Tilman, D. In Encyclopaedia of Biodiversity (ed. Levin, S. A.) 109–120 (Academic, 2001).
Santini, L. et al. Assessing the suitability of diversity metrics to detect biodiversity change. Biol. Conserv. 213 , 341–350 (2017).
Tumolo, B. B. et al. Toward spatio‐temporal delineation of positive interactions in ecology. Ecol. Evol. 10 , 9026–9036 (2020).
Article PubMed PubMed Central Google Scholar
Blowes, S. A. et al. The geography of biodiversity change in marine and terrestrial assemblages. Science 366 , 339–345 (2019).
van Klink, R. et al. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368 , 417–420 (2020).
Article ADS PubMed Google Scholar
Pilotto, F. et al. Meta-analysis of multidecadal biodiversity trends in Europe. Nat. Commun. 11 , 3486 (2020).
Bouraoui, F. & Grizzetti, B. Long term change of nutrient concentrations of rivers discharging in European seas. Sci. Total Environ. 409 , 4899–4916 (2011).
Haase, P. et al. Moderate warming over the past 25 years has already reorganized stream invertebrate communities. Sci. Total Environ. 658 , 1531–1538 (2019).
Baker, N. J., Pilotto, F., Jourdan, J., Beudert, B. & Haase, P. Recovery from air pollution and subsequent acidification masks the effects of climate change on a freshwater macroinvertebrate community. Sci. Total Environ. 758 , 143685 (2021).
Eriksen, T. E. et al. A global perspective on the application of riverine macroinvertebrates as biological indicators in Africa, South-Central America, Mexico and Southern Asia. Ecol. Indic. 126 , 107609 (2021).
Dornelas, M. et al. BioTIME: a database of biodiversity time series for the Anthropocene. Glob. Ecol. Biogeogr. 27 , 760–786 (2018).
Clark, T. J. & Luis, A. D. Nonlinear population dynamics are ubiquitous in animals. Nat. Ecol. Evol. 4 , 75–81 (2020).
Article CAS PubMed Google Scholar
McGill, B., Enquist, B., Weiher, E. & Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21 , 178–185 (2006).
McGill, B. J., Dornelas, M., Gotelli, N. J. & Magurran, A. E. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 30 , 104–113 (2015).
Jarzyna, M. A. & Jetz, W. A near half‐century of temporal change in different facets of avian diversity. Glob. Change Biol. 23 , 2999–3011 (2017).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105 , 6668–6672 (2008).
Isaak, D. J. et al. Slow climate velocities of mountain streams portend their role as refugia for cold-water biodiversity. Proc. Natl Acad. Sci. USA 113 , 4374–4379 (2016).
Zarfl, C., Lumsdon, A. E., Berlekamp, J., Tydecks, L. & Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 77 , 161–170 (2015).
Cid, N. et al. From meta‐system theory to the sustainable management of rivers in the Anthropocene. Front. Ecol. Environ. 20 , 49–57 (2022).
Wang, J. et al. What explains the variation in dam impacts on riverine macroinvertebrates? A global quantitative synthesis. Environ. Res. Lett. 15 , 124028 (2020).
Article ADS CAS Google Scholar
Rosset, V. et al. Is eutrophication really a major impairment for small waterbody biodiversity? J. Appl. Ecol. 51 , 415–425 (2014).
Bruno, D. et al. Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments. Glob. Change Biol. 25 , 1612–1628 (2019).
Gebauer, R. et al. Distribution of alien animal species richness in the Czech Republic. Ecol. Evol. 8 , 4455–4464 (2018).
Whelan, M. J. et al. Is water quality in British rivers “better than at any time since the end of the Industrial Revolution”? Sci. Total Environ. 843 , 157014 (2022).
Belletti, B. et al. More than one million barriers fragment Europe’s rivers. Nature 588 , 436–441 (2020).
Durance, I. & Ormerod, S. J. Trends in water quality and discharge confound long-term warming effects on river macroinvertebrates. Freshw. Biol. 54 , 388–405 (2009).
Article CAS Google Scholar
Wood, P. J. & Armitage, P. D. Biological effects of fine sediment in the lotic environment. Environ. Manage. 21 , 203–217 (1997).
Lemm, J. U. et al. Multiple stressors determine river ecological status at the European scale: towards an integrated understanding of river status deterioration. Glob. Change Biol. 27 , 1962–1975 (2021).
Thorslund, J., Bierkens, M. F. P., Oude Essink, G. H. P., Sutanudjaja, E. H. & van Vliet, M. T. H. Common irrigation drivers of freshwater salinisation in river basins worldwide. Nat. Commun. 12 , 4232 (2021).
Verdonschot, R. C. M., Kail, J., McKie, B. G. & Verdonschot, P. F. M. The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 769 , 55–66 (2016).
Romero, G. Q. et al. Pervasive decline of subtropical aquatic insects over 20 years driven by water transparency, non-native fish and stoichiometric imbalance. Biol. Lett. 17 , 20210137 (2021).
Feio, M. J., Dolédec, S. & Graça, M. A. S. Human disturbance affects the long-term spatial synchrony of freshwater invertebrate communities. Environ. Pollut. 196 , 300–308 (2015).
Malaj, E. et al. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl Acad. Sci. USA 111 , 9549–9554 (2014).
Jourdan, J. et al. Reintroduction of freshwater macroinvertebrates: challenges and opportunities. Biol. Rev. 94 , 368–387 (2019).
Bhide, S. V. et al. Addressing the contribution of indirect potable reuse to inland freshwater salinization. Nat. Sustain. 4 , 699–707 (2021).
Maasri, A. et al. A global agenda for advancing freshwater biodiversity research. Ecol. Lett. 25 , 255–263 (2022).
Haase, P. et al. The next generation of site-based long-term ecological monitoring: Linking essential biodiversity variables and ecosystem integrity. Sci. Total Environ. 613–614 , 1376–1384 (2018).
Heino, J. et al. Abruptly and irreversibly changing Arctic freshwaters urgently require standardized monitoring. J. Appl. Ecol. 57 , 1192–1198 (2020).
Didham, R. K. et al. Interpreting insect declines: seven challenges and a way forward. Insect Conserv. Divers. 13 , 103–114 (2020).
Outhwaite, C. L., Gregory, R. D., Chandler, R. E., Collen, B. & Isaac, N. J. B. Complex long-term biodiversity change among invertebrates, bryophytes and lichens. Nat. Ecol. Evol. 4 , 384–392 (2020).
Pandolfi, J. M., Staples, T. L. & Kiessling, W. Increased extinction in the emergence of novel ecological communities. Science 370 , 220–222 (2020).
Arneth, A. et al. Post-2020 biodiversity targets need to embrace climate change. Proc. Natl Acad. Sci. USA 117 , 30882–30891 (2020).
Chapman, D. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring 2nd edn (Taylor & Francis, 1996).
Hallett, L. et al. codyn: community dynamics metrics. R package version 2.0.5 (2020).
Oksanen, A. J. et al. vegan: community ecology package. R package version 2.5-7 (2020).
Schmidt-Kloiber, A. & Hering, D. www.freshwaterecology.info—an online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol. Indic. 53 , 271–282 (2015).
Sarremejane, R. et al. DISPERSE, a trait database to assess the dispersal potential of European aquatic macroinvertebrates. Sci. Data 7 , 386 (2020).
Schmera, D., Heino, J., Podani, J., Erős, T. & Dolédec, S. Functional diversity: a review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 787 , 27–44 (2017).
Schmera, D., Heino, J. & Podani, J. Characterising functional strategies and trait space of freshwater macroinvertebrates. Sci. Rep. 12 , 12283 (2022).
Tachet, H., Richoux, P., Bournaud, M. & Usseglio‐Polatera, P. Invertébrés d’Eau Douce: Systématique, Biologie, Écologie (CNRS Editions, 2010).
Chevenet, F., Dolédec, S. & Chessel, D. A fuzzy coding approach for the analysis of long-term ecological data. Freshw. Biol. 31 , 295–309 (1994).
Kunz, S. et al. Tackling inconsistencies among freshwater invertebrate trait databases: harmonising across continents and aggregating taxonomic resolution. Freshw. Biol. 67 , 275–291 (2022).
Laliberté, E., Legendre, P. & Shipley, B. FD: measuring functional diversity (FD) from multiple traits, and other tools for functional ecology. R package version 1.0-12 (2014).
Mouillot, D. et al. The dimensionality and structure of species trait spaces. Ecol. Lett. 24 , 1988–2009 (2021).
Baker, N. J., Pilotto, F., Haubrock, P. J., Beudert, B. & Haase, P. Multidecadal changes in functional diversity lag behind the recovery of taxonomic diversity. Ecol. Evol. 11 , 17471–17484 (2021).
Pavoine, S. adiv: an R package to analyse biodiversity in ecology. R package version 2.0.1 (2020).
Ricotta, C. et al. Measuring the functional redundancy of biological communities: a quantitative guide. Methods Ecol. Evol. 7 , 1386–1395 (2016).
Roy D. et al. Inventory of alien invasive species in Europe (DAISIE). Figshare https://doi.org/10.15468/ybwd3x (2020).
Seebans, H. Alien species first records database (GAFRD). Figshare https://doi.org/10.5281/zenodo.4632335 (2021).
Seebens, H. et al. Global rise in emerging alien species results from increased accessibility of new source pools. Proc. Natl Acad. Sci. USA 115 , E2264–E2273 (2018).
Article CAS PubMed PubMed Central Google Scholar
GBIF: The Global Biodiversity Information Facility , https://www.gbif.org/ (GBIF, 2022, accessed January 2021).
Yamazaki, D. et al. MERIT Hydro: a high‐resolution global hydrography map based on latest topography dataset. Water Resour. Res. 55 , 5053–5073 (2019).
Amatulli, G. et al. Hydrography90m: a new high-resolution global hydrographic dataset. Earth Syst. Sci. Data 14 , 4525–4550 (2022).
Neteler, M., Bowman, M. H., Landa, M. & Metz, M. GRASS GIS: a multi-purpose open source GIS. Environ. Model. Softw. 31 , 124–130 (2012).
Lehner, B. et al. High‐resolution mapping of the world’s reservoirs and dams for sustainable river‐flow management. Front. Ecol. Environ. 9 , 494–502 (2011).
Abatzoglou, J. T., Dobrowski, S. Z., Parks, S. A. & Hegewisch, K. C. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 5 , 170191 (2018).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using stan. R package version 2.16.3 (2021).
Land Cover CCI Product User Guide Version 2 , https://maps.elie.ucl.ac.be/CCI/viewer/download/ESACCI-LC-Ph2-PUGv2_2.0.pdf (European Space Agency, 2017).
Ziebarth, N. L., Abbott, K. C. & Ives, A. R. Weak population regulation in ecological time series. Ecol. Lett. 13 , 21–31 (2010).
White, E. R. Minimum time required to detect population trends: the need for long-term monitoring programs. Bioscience 69 , 40–46 (2019).
Cusser, S., Helms, J., Bahlia, C. A. & Haddad, N. M. How long do population level field experiments need to be? Utilising data from the 40-year-old LTER network. Ecol. Lett. 24 , 1103–1111 (2021).
Arel-Bundock, V., Diniz, M. A., Greifer, N. & Bacher, E. marginaleffects: predictions, comparisons, slopes, marginal means, and hypothesis tests. R package version 4.2.1 (2023).
Kéry, M. & Schaub, M. Bayesian Population Analysis using WinBUGS: a Hierarchical Perspective (Elsevier, 2012).
Vehtari, A. et al. loo: efficient leave-one-out cross-validation and WAIC for Bayesian models. R package version 2.4.1 (2020).
Download references
Acknowledgements
N. Kaffenberger helped with initial data compilation. Funding for authors and data collection and processing was provided by the EU Horizon 2020 project eLTER PLUS (grant agreement no. 871128); the German Federal Ministry of Education and Research (BMBF; 033W034A); the German Research Foundation (DFG FZT 118, 202548816); Czech Republic project no. P505-20-17305S; the Leibniz Competition (J45/2018, P74/2018); the Spanish Ministerio de Economía, Industria y Competitividad—Agencia Estatal de Investigación and the European Regional Development Fund (MECODISPER project CTM 2017-89295-P); Ramón y Cajal contracts and the project funded by the Spanish Ministry of Science and Innovation (RYC2019-027446-I, RYC2020-029829-I, PID2020-115830GB-100); the Danish Environment Agency; the Norwegian Environment Agency; SOMINCOR—Lundin mining & FCT—Fundação para a Ciência e Tecnologia, Portugal; the Swedish University of Agricultural Sciences; the Swiss National Science Foundation (grant PP00P3_179089); the EU LIFE programme (DIVAQUA project, LIFE18 NAT/ES/000121); the UK Natural Environment Research Council (GLiTRS project NE/V006886/1 and NE/R016429/1 as part of the UK-SCAPE programme); the Autonomous Province of Bolzano (Italy); and the Estonian Research Council (grant no. PRG1266), Estonian National Program ‘Humanitarian and natural science collections’. The Environment Agency of England, the Scottish Environmental Protection Agency and Natural Resources Wales provided publicly available data. We acknowledge the members of the Flanders Environment Agency for providing data. This article is a contribution of the Alliance for Freshwater Life ( www.allianceforfreshwaterlife.org ).
Author information
These authors contributed equally: Peter Haase, Ellen A. R. Welti
Authors and Affiliations
Department of River Ecology and Conservation, Senckenberg Research Institute and Natural History Museum Frankfurt, Gelnhausen, Germany
Peter Haase, Nathan J. Baker & Ellen A. R. Welti
Faculty of Biology, University of Duisburg-Essen, Essen, Germany
Peter Haase, Daniel Hering, Armin W. Lorenz & Stefan Stoll
Department of Ecosystem Services, German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Leipzig, Germany
Diana E. Bowler
Institute of Biodiversity, Friedrich Schiller University Jena, Jena, Germany
Department of Ecosystem Services, Helmholtz Center for Environmental Research—UFZ, Leipzig, Germany
Laboratory of Evolutionary Ecology of Hydrobionts, Nature Research Centre, Vilnius, Lithuania
Nathan J. Baker
FEHM-Lab (Freshwater Ecology, Hydrology and Management), Department of Evolutionary Biology, Ecology and Environmental Sciences, Facultat de Biologia, Institut de Recerca de la Biodiversitat (IRBio), University of Barcelona, Barcelona, Spain
Núria Bonada & Miguel Cañedo-Argüelles
Department of Community and Ecosystem Ecology, Leibniz Institute of Freshwater Ecology and Inland Fisheries (IGB), Berlin, Germany
Sami Domisch, Jaime R. Garcia Marquez, Sonja C. Jähnig, Mathieu Floury, Jens Kiesel & Longzhu Q. Shen
Geography Research Unit, University of Oulu, Oulu, Finland
Geography Department, Humboldt-Universität zu Berlin, Berlin, Germany
Sonja C. Jähnig
Department of Water, Atmosphere and Environment, Institute of Hydrobiology and Aquatic Ecosystem Management, University of Natural Resources and Life Sciences, Vienna, Austria
Astrid Schmidt-Kloiber, Wolfram Graf & Patrick Leitner
School of Science and Technology, Nottingham Trent University, Nottingham, UK
Rachel Stubbington
Department of Evolutionary Biology and Environmental Studies, University of Zurich, Zurich, Switzerland
Florian Altermatt
Department of Aquatic Ecology, Eawag: Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
IHCantabria—Instituto de Hidráulica Ambiental de la Universidad de Cantabria, Santander, Spain
Mario Álvarez-Cabria, José Barquín Ortiz & Francisco J. Peñas
School of the Environment, Yale University, New Haven, CT, USA
Giuseppe Amatulli
Department of Aquatic Sciences and Assessment, Swedish University of Agricultural Sciences, Uppsala, Sweden
David G. Angeler, Richard K. Johnson & Brendan G. McKie
IMPACT, The Institute for Mental and Physical Health and Clinical Translation, Deakin University, Geelong, Victoria, Australia
David G. Angeler
Brain Capital Alliance, San Francisco, CA, USA
School of Natural Resources, University of Nebraska-Lincoln, Lincoln, NE, USA
INRAE, UMR RECOVER Aix Marseille Univ, Centre d’Aix-en-Provence, Aix-en-Provence, France
Gaït Archambaud-Suard & Marie-Helène Lizée
Agencia Vasca del Agua, Vitoria-Gasteiz, Spain
Iñaki Arrate Jorrín & Jesús Alberto Manzanos Arnaiz
Wessex Water, Bath, UK
Thomas Aspin & Andy House
Ekolur Asesoría Ambiental SLL, Oiartzun, Spain
Iker Azpiroz & Manu Rubio
Departamento de Medio Ambiente y Obras Hidráulicas, Diputación Foral de Gipuzkoa, Donostia-San Sebastián, Spain
Iñaki Bañares
LFI—The Laboratory for Freshwater Ecology and Inland Fisheries, NORCE Norwegian Research Centre, Bergen, Norway
Christian L. Bodin & Gaute Velle
Department of Earth and Environmental Sciences—DISAT, University of Milano-Bicocca, Milan, Italy
Luca Bonacina & Riccardo Fornaroli
Institute for Alpine Environment, Eurac Research, Bolzano, Italy
Roberta Bottarin & Alberto Scotti
FEHM-Lab, Institute of Environmental Assessment and Water Research (IDAEA), CSIC, Barcelona, Spain
Miguel Cañedo-Argüelles
Department of Hydrobiology, University of Pécs, Pécs, Hungary
Zoltán Csabai
Department of Botany and Zoology, Faculty of Science, Masaryk University, Brno, Czech Republic
Zoltán Csabai, Petr Paril, Marek Polášek & Michal Straka
INRAE, UR RiverLy, Centre de Lyon-Villeurbanne, Villeurbanne, France
Thibault Datry, Martial Ferréol & Maxence Forcellini
Fisheries Ecosystems Advisory Services, Marine Institute, Newport, Ireland
Elvira de Eyto
Environmental Research and Innovation Department, Luxembourg Institute of Science and Technology, Esch-sur-Alzette, Luxembourg
Alain Dohet & Lionel L’Hoste
Water Development Department, Ministry of Agriculture, Rural Development and Environment, Nicosia, Cyprus
Gerald Dörflinger & Iakovos Tziortzis
Centre for Freshwater and Environmental Studies, Dundalk Institute of Technology, Dundalk, Ireland
Emma Drohan
Norwegian Institute for Nature Research (NINA), Oslo, Norway
Knut A. Eikland, Thomas C. Jensen, Francesca Pilotto & Leonard Sandin
Environment Agency, Wallingford, UK
Judy England
Norwegian Institute for Water Research, Oslo, Norway
Tor E. Eriksen, Nikolai Friberg & Jes Jessen Rasmussen
Department of Aquatic Ecosystems, Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, Bulgaria
Vesela Evtimova, Galia Georgieva, Violeta G. Tyufekchieva, Yordan Uzunov, Emilia Varadinova & Yanka Vidinova
Department of Life Sciences, University of Coimbra, Marine and Environmental Sciences Centre, ARNET, Coimbra, Portugal
Maria J. Feio & Manuel A. S. Graça
Univ Lyon, Université Claude Bernard Lyon 1, CNRS, ENTPE, UMR 5023 LEHNA, Villeurbanne, France
Mathieu Floury
Department of Animal Sciences and Aquatic Ecology, Ghent University, Ghent, Belgium
Marie Anne Eurie Forio, Peter Goethals & Rudy Vannevel
Freshwater Biological Section, University of Copenhagen, Copenhagen, Denmark
Nikolai Friberg
water@leeds, School of Geography, University of Leeds, Leeds, UK
ARALEP—Ecologie des Eaux Douces, Villeurbanne, France
Jean-François Fruget
Department of Ecology and Genetics, University of Oulu, Oulu, Finland
Kaisa-Leena Huttunen & Timo Muotka
School of Biological and Behavioural Sciences, Queen Mary University of London, London, UK
J. Iwan Jones & John F. Murphy
Department of Hydrology and Water Resources Management, Christian-Albrechts-University Kiel, Institute for Natural Resource Conservation, Kiel, Germany
Jens Kiesel
Department of Forest Ecology and Management, Swedish University of Agricultural Sciences, Umeå, Sweden
Lenka Kuglerová
Department of Plant Biology and Ecology, University of the Basque Country, Leioa, Spain
Aitor Larrañaga
Laboratoire National d’Hydraulique et Environnement, EDF Recherche et Développement, Chatou, France
Anthony Maire
Department of Ecology and Hydrology, University of Murcia, Murcia, Spain
Andrés Millán & David Sánchez-Fernández
UK Centre for Ecology & Hydrology, Lancaster Environment Centre, Lancaster, UK
Don Monteith
Institute of Biology, University of Latvia, Riga, Latvia
Davis Ozolins & Agnija Skuja
Oulanka Research Station, University of Oulu Infrastructure Platform, Kuusamo, Finland
Riku Paavola
Institute for Environmental Science, RPTU Kaiserslautern-Landau, Landau, Germany
Ralf B. Schäfer
APEM, Stockport, UK
Alberto Scotti
Institute for Green Science, Carnegie Mellon University, Pittsburgh, PA, USA
Longzhu Q. Shen
Department of Environmental Planning / Environmental Technology, University of Applied Sciences Trier, Birkenfeld, Germany
Stefan Stoll
T.G. Masaryk Water Research Institute, Brno, Czech Republic
Michal Straka
Chair of Hydrobiology and Fishery, Centre for Limnology, Estonian University of Life Sciences, Elva vald, Estonia
Wageningen Environmental Research, Wageningen University and Research, Wageningen, The Netherlands
Gea H. van der Lee, Piet F. M. Verdonschot & Ralf C. M. Verdonschot
Flanders Environment Agency, Aalst, Belgium
Rudy Vannevel
Department of Geography, Ecology and Environment Protection, Faculty of Mathematics and Natural Sciences, South-West University ‘Neofit Rilski’, Blagoevgrad, Bulgaria
Emilia Varadinova
Department of Tisza River Research, Centre for Ecological Research, Institute of Aquatic Ecology, Debrecen, Hungary
Gábor Várbíró
Department of Biological Sciences, University of Bergen, Bergen, Norway
Gaute Velle
Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam, The Netherlands
Piet F. M. Verdonschot
Department of Ecoscience, Aarhus University, Aarhus, Denmark
Peter Wiberg-Larsen
Conservation Ecology Center, Smithsonian National Zoo and Conservation Biology Institute, Front Royal, VA, USA
Ellen A. R. Welti
You can also search for this author in PubMed Google Scholar
Contributions
P.H. conceived the study. N.B., J.H., D.H., S.C.J. and A.S.-K. contributed to initial ideas. E.A.R.W. performed data cleaning. E.A.R.W., D.E.B. and N.J.B. designed and conducted analyses. S.D. and J.R.G.M. provided environmental data with assistance from G.A., J.K. and L.Q.S. E.A.R.W. and P.H. wrote the first draft with assistance from D.E.B., and all of the authors contributed to additional versions. F.A., M.Á.-C., D.G.A., G.A., I.A.J., T.A., I.A., I.B., J.B.O., C.L.B., L.B., N.B., R.B., M.C.-A., N.J.B., Z.C., T.D., E.d.E., A.D., G.D., E.D., K.A.E., J.E., T.E.E., V.E., M.J.F., M. Ferréol, M. Floury, M. Forcellini, M.A.E.F., R.F., N.F., J.-F.F., G.G., P.G., M.A.S.G., W.G., A.H., P.H., K.-L.H., T.C.J., R.K.J., J.I.J., L.K., A.L., P.L., L.L. M.-H.L., A.W.L., A. Maire, J.A.M.A., B.G.M., A. Millán, D.M., T.M., J.F.M., D.O., R.P., F.J.P., F.P., M.P., P.P., J.J.R., M.R., D.S.-F., L.S., R.B.S., A.S.-K., A. Scotti, A. Skuja, S.S., M.S., R. Stubbington, H.T., V.G.T., I.T., Y.U., G.H.v.d.L., R.V., E.V., G. Várbíró, G. Velle, P.F.M.V., R.C.M.V., Y.V. and P.W.-L. provided invertebrate data.
Corresponding authors
Correspondence to Peter Haase or Ellen A. R. Welti .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature thanks Christopher Daly, Yanan Fan, Ian Vaughan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 trend estimates for community subsets..
Overall estimates and distributions of trends in a , non-native species richness, b , non-native abundance, c , native taxon richness, d , native abundance, e , Ephemeroptera, Plecoptera, and Trichoptera (EPT) taxon richness, f , EPT abundance, g , insect taxon richness, and h , insect abundance. Bars around estimates indicate 80%, 90%, and 95% credible intervals. Trend estimates for native taxa (c, d) are restricted to the 1,299 sites at which taxa were identified to species or a mixed taxonomic resolution. Trend estimates for non-native species (a, b) are restricted to the 898 (of 1,299) sites at which non-native species were detected. Incorporating the remaining 394 (30.1%) of the 1,299 sites (i.e. those with no detected non-native species) as having trends = 0 resulted in an average increase of 2.75% y −1 in richness and 2.79% y −1 in abundance.
Extended Data Fig. 2 Trend estimates for additional biodiversity metrics.
Overall estimates and distributions of trends in a , Shannon’s diversity, b , rarefied taxon richness, c , functional divergence, and d , Rao’s quadratic entropy (n = 1,816 biologically independent sites for all metrics). Bars around estimates indicate 80%, 90%, and 95% credible intervals.
Extended Data Fig. 3 Moving window trends for additional biodiversity metrics.
Estimated trends in a , Shannon’s evenness, b , taxonomic turnover, c , functional evenness, and d , functional temporal turnover. Estimates were calculated from Bayesian mixed-effects models of trends from ≥250 time series with ≥6 years of data from ≥8 countries within 10-year moving windows. Grey polygons indicate 80, 90, and 95% credible intervals.
Extended Data Fig. 4 Estimated effects of environmental drivers on temporal trends in additional biodiversity metrics.
Estimated effects of the mean (tmax mean) and trend (tmax sl. [slope]) of annual maximum temperature, mean (ppt mean) and trend (ppt sl.) of annual precipitation, dam impacts (dam), and the percentage of the upstream catchment covered by urban areas and cropland on temporal trends in a , Shannon’s diversity, b , rarefied taxon richness, c , functional (func.) divergence, and d , Rao’s quadratic entropy (Q) (n = 1,816 biologically independent sites for all metrics). Bars around estimates indicate 80%, 90%, and 95% credible intervals. Grey, horizontal lines separate the three environmental driver groups: climate, dams, and land use.
Extended Data Fig. 5 Estimated effects of environmental drivers on biodiversity metrics representing community subsets.
Estimated effects of the mean (tmax mean) and trend (tmax sl. [slope]) of annual maximum temperature, mean (ppt mean) and trend (ppt sl. [slope]) of annual precipitation, dam impacts (dam), and the percentage of the upstream catchment covered by urban areas and cropland on temporal trends in a , non-native species richness, b , native taxon richness, c , EPT richness, d , insect richness, e , non-native abundance, f , native abundance, g , EPT abundance, and h , insect abundance. Trend estimates for native taxa (b, f) are restricted to 1,299 sites at which taxa were identified to species or a mixed taxonomic resolution. Trend estimates for non-native species (a, e) are restricted to the 898 (of 1,299) sites at which non-native species were detected. Bars around estimates indicate 80%, 90%, and 95% credible intervals. Bars around estimates indicate 80%, 90%, and 95% credible intervals. Grey, horizontal lines separate the three environmental driver groups: climate, hydrology, and land use.
Extended Data Fig. 6 Estimated effects of stream characteristics on biodiversity metrics.
Estimated effects of slope, elevation, flow accumulation (accum.) and Strahler stream order (str. order) on temporal trends in a , taxon richness, b , abundance, c , evenness, d , turnover, and functional (func.) e , richness, f , redundancy, g , evenness, and h , turnover (n = 1,816 biologically independent sites for all metrics). Bars around estimates indicate 80%, 90%, and 95% credible intervals.
Extended Data Fig. 7 Estimated effects of stream characteristics on additional biodiversity metrics.
Estimated effects of stream characteristics of slope, elevation, flow accumulation (accum.) and Strahler stream order (str. order) on temporal trends in a , Shannon’s diversity, b , rarefied taxon richness, c , functional (func.) divergence, and d , Rao’s quadratic entropy (n = 1,816 biologically independent sites for all metrics). Bars around estimates indicate 80%, 90%, and 95% credible intervals.
Extended Data Fig. 8 Estimated effects of stream characteristics on taxon richness and abundance of taxa subsets.
Estimated effects of slope, elevation, flow accumulation (accum.) and Strahler stream order (str. order) on temporal trends in a , non-native species richness, b , native taxon richness, c , EPT richness, d , insect richness, e , non-native abundance, f , native abundance, g , EPT abundance, and h , insect abundance. Trend estimates for native taxa (b, f) are restricted to 1,299 sites at which taxa were identified to species or a mixed taxonomic resolution. Trend estimates for non-native species (a, e) are restricted to the 898 (of 1,299) sites at which non-native species were detected. Bars around estimates indicate 80%, 90%, and 95% credible intervals.
Extended Data Fig. 9 Sensitivity of biodiversity metric responses to taxonomic identification level.
Error bars represent 95% credible intervals. Overlapping error bars indicate comparable trend estimates for analyses at species (n = 762), genus/mixed (n = 537) and family (n = 517) taxonomic levels; Func., functional; Est., estimated trend.
Extended Data Fig. 10 Sensitivity of biodiversity metric responses to sampling season.
Error bars represent 95% credible intervals. The largest differences between seasons were found for winter, which likely reflects the low number of sites sampled in this season (winter n = 5, spring n = 623, summer n = 473, fall n = 715). Func. refers to functional; Est. refers to trend estimates.
Supplementary information
Supplementary information.
Supplementary equation (1) and Supplementary Tables 1–6
Reporting Summary
Peer review file, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Haase, P., Bowler, D.E., Baker, N.J. et al. The recovery of European freshwater biodiversity has come to a halt. Nature 620 , 582–588 (2023). https://doi.org/10.1038/s41586-023-06400-1
Download citation
Received : 24 March 2022
Accepted : 04 July 2023
Published : 09 August 2023
Issue Date : 17 August 2023
DOI : https://doi.org/10.1038/s41586-023-06400-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The need of centralized coordination to counter biological invasions in the european union.
- Irmak Kurtul
- Phillip J. Haubrock
Environmental Sciences Europe (2024)
Lake microbiome composition determines community adaptability to warming perturbations
- Xiaotong Wu
- Qixing Zhou
- Xiangang Hu
Ecological Processes (2024)

Are long-term biomonitoring efforts overlooking crayfish in European rivers?
- Ismael Soto
- Antonín Kouba
Revealing uncertainty in the status of biodiversity change
- T. F. Johnson
- A. P. Beckerman
- R. P. Freckleton
Nature (2024)
Long-term wetland biomonitoring highlights the differential impact of land use on macroinvertebrate diversity in Dongting Lake in China
- Daizhong Huang
- Xiaowei Zhang
Communications Earth & Environment (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Ecological monitoring, assessment, and management in freshwater systems.

1. Introduction
2. contributions, 3. conclusions, acknowledgments, author contributions, conflicts of interest.
- Park, Y.-S. Aquatic Ecosystem assessment and management. Ann. Limnol. Int. J. Limnol. 2016 , 52 , 61–63. [ Google Scholar ] [ CrossRef ]
- Park, Y.-S.; Chon, T.-S. Editorial: Ecosystem assessment and management. Ecol. Inform. 2015 , 29 , 93–95. [ Google Scholar ] [ CrossRef ]
- Water Framework Directive, European Union (WFD E.U.). Establishing a Framework for Community Action in the Field of Water Policy ; Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000; European Union (EU): Brussels, Belgium, 2000. [ Google Scholar ]
- Arle, J.; Mohaupt, V.; Kirst, I. Monitoring of Surface Waters in Germany under the Water Framework Directive—A Review of Approaches, Methods and Results. Water 2016 , 8 , 217. [ Google Scholar ] [ CrossRef ]
- Buss, D.F.; Carlisle, D.M.; Chon, T.-S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Robinson, W.A.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream biomonitoring using macroinvertebrates around the globe: A comparison of large-scale programs. Environ. Monit. Assess. 2015 , 187 , 4132. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Qu, X. Personal Communication, Institute of Water Resource and Hydropower Research: Beijing, China, 2016.
- Hwang, S.J.; Lee, S.-W.; Park, Y.-S. Editorial: Ecological monitoring, assessment, and restoration of running waters in Korea. Ann. Limnol. Int. J. Limnol. 2011 , 47 , S1–S2. [ Google Scholar ] [ CrossRef ]
- Lee, S.-W.; Hwang, S.-J.; Lee, J.-K.; Jung, D.-I.; Park, Y.-J.; Kim, J.-T. Overview and application of the National Aquatic Ecological Monitoring Program (NAEMP) in Korea. Ann. Limnol. Int. J. Limnol. 2011 , 47 , S3–S14. [ Google Scholar ] [ CrossRef ]
- Jiang, M.; Jeong, K.; Park, J.-H.; Kim, N.-Y.; Hwang, S.-J.; Kim, S.-H. Open, Sharable, and Extensible Data Management for the Korea National Aquatic Ecological Monitoring and Assessment Program: A RESTful API-Based Approach. Water 2016 , 8 , 201. [ Google Scholar ] [ CrossRef ]
- Choi, B.; Choi, S.-U.; Kang, H. Transferability of Monitoring Data from Neighboring Streams in a Physical Habitat Simulation. Water 2015 , 7 , 4537–4551. [ Google Scholar ] [ CrossRef ]
- Li, B.; Watanabe, K.; Kim, D.-H.; Lee, S.-B.; Heo, M.; Kim, H.-S.; Chon, T.-S. Identification of Outlier Loci Responding to Anthropogenic and Natural Selection Pressure in Stream Insects Based on a Self-Organizing Map. Water 2016 , 8 , 188. [ Google Scholar ] [ CrossRef ]
- Park, Y.-S.; Song, M.-Y.; Park, Y.-C.; Oh, K.-H.; Cho, E.; Chon, T.-S. Community patterns of benthic macroinvertebrates collected on the national scale in Korea. Ecol. Model. 2007 , 203 , 26–33. [ Google Scholar ] [ CrossRef ]
- Bae, M.-J.; Li, F.; Kwon, Y.-S.; Chung, N.; Choi, H.; Hwang, S.-J.; Park, Y.-S. Concordance of diatom, macroinvertebrate and fish assemblages in streams at nested spatial scales: Implications for ecological integrity. Ecol. Indic. 2014 , 47 , 89–101. [ Google Scholar ] [ CrossRef ]
- Jun, Y.-C.; Kim, N.-Y.; Kim, S.-H.; Park, Y.-S.; Kong, D.-S.; Hwang, S.-J. Spatial Distribution of Benthic Macroinvertebrate Assemblages in Relation to Environmental Variables in Korean Nationwide Streams. Water 2016 , 8 , 27. [ Google Scholar ] [ CrossRef ]
- Grygoruk, M.; Frąk, M.; Chmielewski, A. Agricultural Rivers at Risk: Dredging Results in a Loss of Macroinvertebrates. Preliminary Observations from the Narew Catchment, Poland. Water 2015 , 7 , 4511–4522. [ Google Scholar ] [ CrossRef ]
- Meyer, J.L.; Strayer, D.L.; Wallace, J.B.; Eggert, S.L.; Helfman, G.S.; Leonard, N.E. The contribution of headwater streams to biodiversity in river networks. J. Am. Water Resour. Assoc. 2007 , 43 , 86–103. [ Google Scholar ] [ CrossRef ]
- Bae, M.-J.; Chun, J.H.; Chon, T.-S.; Park, Y.-S. Spatio-Temporal Variability in Benthic Macroinvertebrate Communities in Headwater Streams in South Korea. Water 2016 , 8 , 99. [ Google Scholar ] [ CrossRef ]
- Lee, H.-J.; Chun, K.-W.; Shope, C.L.; Park, J.-H. Multiple Time-Scale Monitoring to Address Dynamic Seasonality and Storm Pulses of Stream Water Quality in Mountainous Watersheds. Water 2015 , 7 , 6117–6138. [ Google Scholar ] [ CrossRef ]
- Wang, H.; Chen, Y.; Liu, Z.; Zhu, D. Effects of the “Run-of-River” Hydro Scheme on Macroinvertebrate Communities and Habitat Conditions in a Mountain River of Northeastern China. Water 2016 , 8 , 31. [ Google Scholar ] [ CrossRef ]
- Kim, D.-H.; Chon, T.-S.; Kwak, G.-S.; Lee, S.-B.; Park, Y.-S. Effects of Land Use Types on Community Structure Patterns of Benthic Macroinvertebrates in Streams of Urban Areas in the South of the Korea Peninsula. Water 2016 , 8 , 187. [ Google Scholar ] [ CrossRef ]
- Hwang, S.-A.; Hwang, S.-J.; Park, S.-R.; Lee, S.-W. Examining the Relationships between Watershed Urban Land Use and Stream Water Quality Using Linear and Generalized Additive Models. Water 2016 , 8 , 155. [ Google Scholar ] [ CrossRef ]
- Yun, Y.-J.; An, K.-G. Roles of N:P Ratios on Trophic Structures and Ecological Stream Health in Lotic Ecosystems. Water 2016 , 8 , 22. [ Google Scholar ] [ CrossRef ]
- An, K.-J.; Lee, S.-W.; Hwang, S.-J.; Park, S.-R.; Hwang, S.-A. Exploring the Non-Stationary Effects of Forests and Developed Land within Watersheds on Biological Indicators of Streams Using Geographically-Weighted Regression. Water 2016 , 8 , 120. [ Google Scholar ] [ CrossRef ]
- Kim, J.Y.; An, K.-G. Integrated Ecological River Health Assessments, Based on Water Chemistry, Physical Habitat Quality and Biological Integrity. Water 2015 , 7 , 6378–6403. [ Google Scholar ] [ CrossRef ]
- Glińska-Lewczuk, K.; Burandt, P.; Kujawa, R.; Kobus, S.; Obolewski, K.; Dunalska, J.; Grabowska, M.; Lew, S.; Chormański, J. Environmental Factors Structuring Fish Communities in Floodplain Lakes of the Undisturbed System of the Biebrza River. Water 2016 , 8 , 146. [ Google Scholar ] [ CrossRef ]
- Kim, J.-H.; Yoon, J.-D.; Baek, S.-H.; Park, S.-H.; Lee, J.-W.; Lee, J.-A.; Jang, M.-H. An Efficiency Analysis of a Nature-Like Fishway for Freshwater Fish Ascending a Large Korean River. Water 2016 , 8 , 3. [ Google Scholar ] [ CrossRef ]
- Drapper, D.; Hornbuckle, A. Field Evaluation of a Stormwater Treatment Train with Pit Baskets and Filter Media Cartridges in Southeast Queensland. Water 2015 , 7 , 4496–4510. [ Google Scholar ] [ CrossRef ]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-S.; Hwang, S.-J. Ecological Monitoring, Assessment, and Management in Freshwater Systems. Water 2016 , 8 , 324. https://doi.org/10.3390/w8080324
Park Y-S, Hwang S-J. Ecological Monitoring, Assessment, and Management in Freshwater Systems. Water . 2016; 8(8):324. https://doi.org/10.3390/w8080324
Park, Young-Seuk, and Soon-Jin Hwang. 2016. "Ecological Monitoring, Assessment, and Management in Freshwater Systems" Water 8, no. 8: 324. https://doi.org/10.3390/w8080324
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10303708

The Global Trend of Microplastic Research in Freshwater Ecosystems
Yaochun wang.
1 Department of Geography and Spatial Information Techniques, Ningbo University, Ningbo 315211, China
2 Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou 510380, China
Naicheng Wu
Associated data.
Not applicable.
The study of microplastics and their impact on aquatic ecosystems has received increasing attention in recent years. Drawing from an analysis of 814 papers related to microplastics published between 2013 and 2022 in the Web of Science Core Repository, this paper explores trends, focal points, and national collaborations in freshwater microplastics research, providing valuable insights for future studies. The findings reveal three distinct stages of microplastics: nascent development (2013–2015), slow rise (2016–2018), and rapid development (2019–2022). Over time, the focus of research has shifted from “surface”, “effect”, “microplastic pollution”, and “tributary” to “toxicity”, “species”, “organism”, “threat”, “risk”, and “ingestion”. While international cooperation has become more prevalent, the extent of collaboration remains limited, mostly concentrated among English-speaking countries or English and Spanish/Portuguese-speaking countries. Future research directions should encompass the bi-directional relationship between microplastics and watershed ecosystems, incorporating chemical and toxicological approaches. Long-term monitoring efforts are crucial to assessing the sustained impacts of microplastics.
1. Introduction
The low decomposition rate and high productivity (300 million tons in 2020 [ 1 ]) of plastic have led to its increased presence in ecosystems, resulting in negative impacts on freshwater bodies [ 2 ]. Plastic pollution was listed as one of the “top 10 urgent environmental issues” by the United Nations Environment Assembly in 2014, and in 2018, World Environment Day was celebrated under the theme of “Plastic Wars Plastic Decisions” [ 3 ]. The occurrence of plastic in water has a toxic effect on organisms and poses significant ecological risks [ 4 ]. It is evident that, amidst the dramatic increase in demand for plastics, finding a balanced solution between environmental friendliness and plastic consumption is of utmost importance.
Plastic particles with a size smaller than 5 mm are commonly referred to as “microplastics” [ 5 , 6 ]. In the aquatic environment, plastic waste undergoes breakdown through mechanical overuse, photodegradation, oxidation, and weathering, resulting in the formation of small particles (<5 mm) known as secondary microplastics. On the other hand, plastic particles present in cosmetics and fibers in clothing are considered primary microplastics. Due to their small size, microplastics can be transported over long distances by wind, rivers, and ocean currents. Numerous studies have confirmed the impact of microplastics on organisms and ecosystems. Available information indicates that microplastic pollution is found in varying abundances across different locations, ranging from the equator [ 7 , 8 ] to the north and south Poles [ 9 , 10 ]. Previous studies have shown significant findings: Wright and Kelly found that the human excretory system eliminates 90% of microplastics through feces, while the remaining 10% is absorbed into the bloodstream [ 11 ]; the same study also revealed that nanoscale microplastics can pass through the brains of carp, leading to behavioral disorders. Additionally, Martins and Guilhermino discovered that microplastics can induce transgenerational effects in Daphnia, resulting in birth disorders and reduced growth rates in their offspring [ 12 ]. However, it is important to note that most toxicity studies have been conducted at high concentrations that are not environmentally relevant and often involve pristine polymers. In summary, global microplastic pollution has the potential to significantly impact ecosystems and human health. Therefore, ecological and environmental studies on microplastics are of great significance [ 13 , 14 ].
The sourcing, storage, pollution, and utilization of freshwater, a vital resource for irrigation and sustaining life on land, constitute a significant global concern [ 15 , 16 , 17 , 18 , 19 ]. Microplastics have been found in rivers and lakes [ 1 ] as well as wetlands [ 20 ]. Several highly cited review articles [ 1 , 20 , 21 ] have made valuable contributions by providing comprehensive insights into the methodologies, characteristics, distribution, and pollution associated with microplastics. However, these reviews primarily rely on traditional approaches such as summarizing the existing literature, which may result in limited coverage of the available research. To address this limitation, an analysis of trends, focal points, and national collaborations in microplastic research specifically focused on freshwater environments can offer a macro-level summary of the current state of knowledge. This approach, based on a comprehensive examination of numerous publications, provides a valuable resource for future research endeavors in the field of microplastics in freshwater environments.
With the increasing number of publications, publishing institutions, and international collaborations, there is a growing need for an objective and macroscopic research methodology. Bibliometrics plays a crucial role in assessing and analyzing researchers’ productivity [ 22 ], inter-institutional collaborations [ 23 ], the impact of national research investments on research and development [ 24 ], and the quality of scholarly work [ 25 ]. By analyzing key information from a large number of publications and utilizing visual methods to represent the intersection, derivation, and interaction between research objects, bibliometrics can help predict trends, explore interrelationships, and uncover hotspots and frontiers in research [ 26 ]. However, few articles have combined bibliometrics and the literature reviews to explore the progress of microplastic research in freshwater ecosystems. Therefore, this paper employs bibliometric analysis to summarize research trends, focal points, and international collaborations in the field of freshwater microplastics. Based on the selected keywords (TI = (microplastic*) AND TI = (fresh water or freshwater or plain water or lake or river or canal or stream or wetland or wetland or glacier or ice or groundwater or groundwater or underground water)) and the defined selection criteria, articles published since 2013 are included in this study. The objectives of this research are as follows: (i) to identify research trends in publications on microplastics in freshwater ecosystems from the period 2013 to 2022; (ii) to identify the focal points of publications on microplastics in freshwater ecosystems from 2013 to 2022; and (iii) to explore the trends of international cooperation on microplastics in freshwater ecosystems from 2013 to 2022.
2. Materials and Methods
2.1. data sources.
Based on the Web of Science Core Collection database (including SCI source journals, SSCI source journals, CCR source journals and CI source journals), publications were searched using the following search term: TI = (microplastic*) AND TI = (freshwater or plain water or lake or river or canal or stream or wetland or glacier or ice or groundwater or underground water) to filter the articles in January 2023. After filtering and eliminating irrelevant literature, 814 articles related to the topic were selected. Since the articles were mainly distributed after 2013, this article focuses on the articles from 2013 to 2022 for analysis. It should be noted that this paper only analyzes articles from the core database and therefore may not include all studies on microplastics in freshwater environments.
2.2. Research Methodology
In this paper, we analyze the research focal points on microplastics in freshwater ecosystems from different periods by combining bibliometrics and the literature reviews. We used R software [ 27 , 28 ] to analyze trends in publication and international cooperation. Additionally, we utilized VOSviewer software [ 29 ] to analyze focal points at different periods. Specifically, we identified high-frequency keywords (focal points) based on the number of occurrences in the article titles and considered collaboration between authors from different countries as international collaboration. Notably, in the analysis of focal points with VOSviewer software, we selected keywords with more than five occurrences in 2013–2017 to be displayed in the figure, and keywords with more than ten occurrences in 2018–2022 to be shown. This selection was necessary due to the large number of keywords, and we focused on displaying high-frequency words. Regarding the analysis of country cooperation, we chose to display collaborations with more than two occurrences in 2013–2017 in the figure, and collaborations with more than five occurrences in 2018–2022.
3.1. Analysis of Publication Numbers
Based on the search results, we analyzed the publication trends of microplastics in freshwater ecosystems ( Figure 1 ). Although the first article on microplastics was published in 1977 [ 30 ], there was limited attention paid to freshwater microplastics before 2013. However, our results demonstrate a steady increase in the number of published articles each year from 2013 to 2022. Based on the annual publication numbers, we divided the research development process into three stages. The first stage, from 2013 to 2015, can be characterized as the nascent development stage, with a small number of papers and a slow rate of increase. The average annual number of publications during this stage was below 5. The second stage, from 2016 to 2018, marked the slow rise stage, with an average of 25 publications per year, resulting in a total of 75 papers. Finally, the period from 2019 to 2022 represented the rapid development stage, with a total of 725 articles published, accounting for 90% of the total literature. The annual rate of increase during this stage exceeded 50%. Overall, there has been a consistent and gradual increase in the number of publications related to microplastics in freshwater ecosystems.

Publications in the field of microplastics in freshwater ecosystems from 2013 to 2022 (data from the Web of Science Core Collection). The horizontal axis represents the different years from 2013-2022 and the vertical axis represents the number of publications. The numbers on the line represent the number of publications corresponding to each year.
3.2. Research Trends
Figure 2 illustrates the most frequently occurring keywords in the titles of the 814 articles and their associations with other keywords. Figure 2 a provides an overview of the overall research focal point and trends from 2013 to 2022. Additionally, Figure 2 b presents the focal points specifically from 2013 to 2017, while Figure 2 c focuses on the period between 2018 and 2022. In the earlier years (2013–2017), the research focal point, as shown in Figure 2 b, exhibited a certain level of repetition. The main areas of focus were “polyethylene”, “surface”, “effect”, “microplastic pollution”, and “tributary”. These 21 keywords can be categorized into three topics: sources of microplastics (green), distribution patterns and methods (blue), and environmental impacts (red).

Research focal points and trends of microplastics in freshwater ecosystems over different periods. ( a ): 2013–2022; ( b ): 2013–2017; ( c ): 2018–2022. The size of the circles indicates the frequency of the keywords; the larger the circle, the higher the frequency of occurrence; the different colors symbolize the hot topics in which the keywords are found, with the keywords at the edges of the hot topics being transitional words; and the lines between the keywords indicate their connections to other keywords.
However, as depicted in Figure 2 c for the years 2018 to 2022, the research focus on microplastics expanded significantly. The emphasis shifted towards “toxicity”, “species”, “organism”, “threat”, “risk”, and “ingestion”. The 42 keywords in this period are grouped into two prominent topics: sources and distribution patterns (green) and environmental impacts (red). Notably, the proportion of environmental impacts increased compared to the earlier years. Overall, the research conducted between 2018 and 2022 exhibited a wider range of focal points and research areas than that of 2013–2017. The research focal point gradually shifted from the characteristics of microplastics to the environmental impacts they pose.
3.3. Trends in International Cooperation
The number of articles published by countries has shown an overall increase, as depicted in Figure 3 and Table 1 (and Supplementary Table S1 and S2 ). International cooperation in microplastics research has also witnessed strengthening, but there are still several countries that have not actively participated in such collaborations, including Sudan, Central Africa, Syria, Myanmar, Afghanistan, and Venezuela. The collaborations that have occurred primarily involve English-speaking countries or English and Spanish/Portuguese-speaking countries. Currently, China, the UK, the US, and Germany exert more influence in microplastic research. Notably, Czechia, France, and Slovenia have produced higher-quality articles, as indicated by their higher average number of citations, during the period of 2013–2017. Similarly, Singapore has shown a similar trend from 2018 to 2022.

Temporal trend changes in international cooperation on microplastics in aquatic ecosystems. ( a ): 2013–2022; ( b ): 2013–2017; ( c ): 2018–2022. The shade of color indicates the number of articles published by countries; the darker the color, the more articles are published; red lines indicate international collaborations; the higher the volume of collaborative publications between two countries, the thicker the red line between them and the closer the relationship (We specify that the display starts after more than five international collaborations in 2013–2017 and after more than ten international collaborations in 2017–2022).
Top 10 cited countries and publications from 2013 to 2022 (publications, number of citations—the total number of citations for articles published in this country).
| 2013–2017 | 2018–2022 | 2013–2022 |
|---|---|---|
| United States (29, 4053) | China (748, 10,571) | China (883, 12,898) |
| United Kingdom (12, 3821) | United Kingdom (91, 2161) | United Kingdom (196, 6054) |
| China (12, 2314) | United States (160, 2001) | United States (122, 5983) |
| Germany (6, 1136) | Germany (143, 1957) | Germany (149, 3093) |
| Canada (17, 1057) | Italy (84, 953) | Canada (89, 1715) |
| Netherlands (6, 923) | Portugal (59, 912) | Netherlands (28, 1636) |
| Switzerland (4, 602) | Spain (63,827) | Italy (93, 1010) |
| France (3, 565) | Singapore (1, 822) | India (81, 1010) |
| Czechia (1, 350) | India (70, 763) | Portugal (67, 914) |
| Slovenia (3, 318) | Australia (52, 736) | France (52, 993) |
Figure 3 a shows the international cooperation in freshwater microplastics research from 2013–2022. Figure 3 b and Supplementary Table S1 highlight that international cooperation in microplastic-related research was minimal from 2013 to 2017. However, over the past five years, international collaboration has increased notably between the Americas and Europe, as depicted in Figure 3 c and Supplementary Table S2 . During the period of 2018–2022, there has been a significant rise in connections between China and the USA, Australia, and Canada. Notably, the US, UK, Australia, and Canada have exhibited greater international activity. Overall, research on freshwater microplastics is increasingly establishing connections across continents, with the most closely linked collaborations involving China and the USA, China and Australia, China and Canada, the USA and Canada, and the USA and the UK.
Table 1 lists the top ten countries with the most cited publications under freshwater microplastics research. From 2013–2017, the United States ranked first in citations (4053), while Czechia had a smaller number of publications but ranked tenth (350) in citations and first in average citations, above the United States. There is little difference in the ranking of the UK, China, Canada, and Germany regarding the number of articles published and citations. Overall, the US had a more substantial influence during this period.
From 2018 to 2022, China has a clear advantage in terms of the number of publications (748) and citations (10,571), with more than four times the number of publications than the second place (2161), but the average citation of publications is relatively low. Singapore has the highest average number of citations (822), a large difference from second place. During the same period, the US ranked second in terms of the number of articles published but third in terms of citations, indicating a decline in the overall influence of the US during this period.
4. Discussion
4.1. sources and distribution patterns of microplastics from 2013 to 2017.
The inclusion of terms such as “polypropylene (PP)” and “polyethylene (PE)”, as well as “polymer type”, from 2013 to 2017 suggests that the distribution of microplastics by polymer type was a major focus during this time period. PP and PE have been identified as primary sources of microplastics in extensive research and have remained significant concerns throughout the history of microplastic studies. PE is widely used in various industrial applications, such as packaging films for industrial and food products, shopping bags, garbage bags, and wrap-around films. Additionally, PP is mainly used in automobiles, appliances, and single-use packaging, with an average of 20% of the plastic parts used in every car worldwide being made of it, making it the most common source of microplastics [ 31 ]. The combined analysis demonstrates a high level of concern for both microplastics and their prevalence in freshwater between 2013 and 2017.
The presence of terms such as “tributary” and “surface” indicates the exploration of microplastic distribution areas from 2013 to 2017. Scholars during this stage have focused on discussing tributaries, rivers [ 32 ], estuaries [ 33 ], lakes [ 34 ], and streams [ 35 ]. Based on our literature summary, many studies during this period (2013–2017) have analyzed the distribution of microplastics in terms of surface water microplastic pollution [ 36 , 37 , 38 ] in relation to their quantity, abundance, shape, and color [ 35 ]. However, the distribution of microplastics can vary in different environments [ 36 ], and it is important to enhance the study of microplastics in different habitats. Zbyszewski and Corcoran suggested that the number and shape of microplastics can indicate whether they have undergone fragmentation, such as being subjected to wave action, sedimentation, or oxidation [ 39 ]. During 2013–2017, the focus was on the morphological characteristics of microplastics and their presence in the water column, although most attention was given to surface waters. Microplastics of different materials have different densities, and therefore, they can be distributed throughout the water column, with low-density microplastics typically occupying the surface water environment while high-density microplastics are often found in sediments and benthic organisms [ 40 ]. However, physical or chemical breakdowns can alter the size and structure of microplastics, thereby changing their position in the water column as a secondary effect [ 40 ].
In summary, the focal point for sources and distribution patterns of microplastics in 2013–2017 is the microplastic distribution and physical characteristics (e.g., number, size, distribution), while studies of environmental impacts are lacking.
4.2. Environmental Impacts from 2013 to 2017
The study of the sources and distribution patterns of microplastics eventually led to a discussion of their impact on water bodies. The inclusion of critical words such as “influence”, “concern”, and “organism” demonstrates the scholarly focus on the effects of microplastics on freshwater. However, the focus on these topics during this stage was not comprehensive and primarily relied on studying their interaction with the environment and organisms based on particle size, material, and shape [ 35 , 41 ] and their sorption effects.
Microplastics in the aquatic environment can directly or indirectly affect the quality of the abiotic environment. Plastics can impact the scattering of light in the aquatic environment, thereby affecting chemical cycling. Different concentrations of microplastics have different effects on the environment [ 42 ]. For example, estuaries with high concentrations of microplastic pollutants have more significant pollution effects [ 42 ]. In addition to interactions with the abiotic environment, microplastics have broader impacts by indirectly or directly affecting biological communities [ 43 ]. Although this topic has attracted some attention since 2013, few scholars focused on it before 2018. Some studies have demonstrated the uptake of microplastics by crabs, amphibians, and invertebrates [ 44 , 45 ]. However, there is uncertainty regarding whether ingestion of uncontaminated microplastics will impact the health of organisms [ 46 ]. Research has primarily focused on whether organisms ingest microplastics, while there is still limited research on the effects after ingestion.
4.3. Sources and Distribution Patterns of Microplastics from 2018 to 2022
More attention has been given to the freshwater environment since 2018, likely due to a greater awareness of its direct connection to human health. Studies conducted in freshwater environments such as the Kelvin River in the UK, Poyang Lake in China, the Lagoon of Bizerte in Tunisia, the Flemish Rivers in Belgium, and Vembanad Lake in India have revealed that microplastic concentrations in the freshwater range from 0.01 to 3 g/L [ 47 , 48 , 49 , 50 ].
The sources of microplastics include “polyvinyl chloride (PVC)”, “polyester”, “pellet”, and “fiber”, with fibrous microplastics being of particular concern from 2018 to 2022. Numerous studies have found high rates of fibrous microplastics in laboratory simulations of laundry processes, as well as in microplastic emissions from wool textiles during home laundering [ 51 , 52 , 53 , 54 ]. Freshwater microplastic samples also exhibited a significant amount of fibrous microplastics, up to 59% [ 55 ]. Polyvinyl chloride (PVC), a highly carcinogenic material capable of adsorbing and accumulating triclosan and affecting zebrafish, has been identified as a source of microplastics in the aquatic environment [ 56 ], leading to increased interest among scholars. PVC and polyester, due to their high densities, tend to sink to the bottom of rivers, while PP and PP are floating microplastics due to their low densities.
The final distribution of microplastics in the water column is also influenced by their degree of weathering, sorption, and rate of aging [ 57 ]. Denser microplastics may cause more lasting damage to the environment. Various environmental factors can affect the rate and mode of microplastic decomposition [ 58 ], and physical breakdown can be slower in freshwater compared to marine environments [ 55 ]. Some studies have shown that certain lake environments may experience more significant weathering of microplastic particles due to enhanced UV penetration [ 38 ]. Additionally, research has started to focus on domestic wastewater. Studying microplastics in domestic wastewater provides insights into the quantities and characteristics of microplastics released through household discharge. Overall, microplastic enrichment is more prominent in areas with intense human activity, and research is beginning to shift toward freshwater and urban environments that are in closer proximity to humans.
4.4. Environmental Impacts from 2018 to 2022
The largest proportion of the environmental impact of microplastics occurred between 2018 and 2022, with a greater focus on topics such as “species”, “transport”, “risk”, “adsorption”, “ingestion”, “interaction”, “threat”, “toxicity”, and “fish” compared to the period of 2013–2017. Toxicological and adsorption effects of microplastics: research on the toxicology of microplastics has primarily concentrated on aquatic organisms, with limited investigation into their effects on humans. It has been discovered that the breakdown of untreated plastic waste in water into microplastics can result in more complex ecological threats as they migrate and adsorb substances [ 59 ]. Numerous scholars have also observed that microplastics combine with harmful environmental pollutants such as additives, pesticides, heavy metals, and pharmaceuticals, forming more complex mixed pollutants. Consequently, the complex contaminants associated with microplastics are linked to various human diseases, including obesity, endocrine disorders, cancer, and cardiovascular problems, indicating that microplastic pollution poses a toxicological threat to human health [ 60 , 61 ].
Overall, the environmental impact of microplastics can pose a more serious toxicological threat, depending on the combination of contaminants. While microplastics tend to absorb pollutants from the environment [ 62 ], their sorption capacity varies among different types and environments [ 63 ]. This variation can be attributed to different microplastics having rubbery domains, functional groups, and polarity. Research suggests that polyethylene (PE) has a more flexible structure compared to other materials, making it more prone to adsorb organic compounds [ 64 , 65 ]. Older microplastics are more likely to adsorb contaminants compared to newly introduced ones, and the polarity of the microplastic and the contaminant during adsorption affects the process. For example, the amide-based polar polymer polyamide (PA) exhibits a higher adsorption capacity for polar antibiotics [ 66 ], while polar polystyrene (PS) has a higher affinity for polar nitrobenzene, and non-polar perfluorooctane sulfonamide (FOSA) has a higher adsorption capacity for non-polar PE [ 67 ].
Additionally, environmental factors such as pH, salinity, and the concentration of contaminants in the surroundings influence the sorption process. Studies have demonstrated that increasing salinity and pH both enhance the sorption capacity of PE [ 68 ]. Interestingly, microplastics sampled closer to the pollution source generally exhibit higher sorption capacities than those collected farther away. In summary, the sorption behavior of microplastics is influenced by both environmental factors and their physical properties. However, the ingestion of such contaminated microplastics by aquatic organisms can result in more severe and widespread harm and may even pose a risk to humans through trophic transfer.
Microplastics have an impact on aquatic organisms and the food web. The inclusion of keywords such as “fish” among the topics indicates that more scholars are beginning to investigate the actual effects of microplastics on organisms. In recent years, there have been an increasing number of experiments simulating the ingestion of microplastics by freshwater organisms to study their presence and effects [ 43 , 69 , 70 ]. These studies have demonstrated that once aquatic organisms ingest microplastics, their digestive systems are the first to be affected. For instance, here’s a study discovered that microplastics accumulate in the digestive tract, causing intestinal blockage, pseudo-satiation, and reduced food capacity [ 71 ]. Studies on crayfish have shown that their feeding rate and body weight decrease with increased ingestion of microplastics [ 72 ], while fish experience intestinal damage, such as rupture of intestinal villi and splitting of intestinal epithelial cells, due to microplastic ingestion [ 73 ]. Persistent damage to the digestive system can lead to more severe consequences, including growth inhibition, damage to the reproductive system, and reduced mobility [ 46 ]. Excessive ingestion of microplastics can also cause other adverse effects, including oxidative stress, altered enzyme levels, and physical organ damage [ 72 , 73 ]. It is important to note that these effects are based on currently observable levels, and microplastics that go unnoticed may gradually break down into smaller particles within the organism, penetrating the cell membranes and participating in the body’s circulation.
As previously discussed, chemically contaminated microplastics will carry toxic substances into organisms and the food chain, damaging the entire food web [ 62 , 74 ]. As early as 2014, some scholars have found that microplastics moved along the food web from lower to higher trophic levels [ 75 ]. In 2016, in the well-known experiment by Batel, an artificial food chain including Artemia to zebrafish was set up and found that microplastics can eventually accumulate in zebrafish via Artemia [ 76 ]. Following this, some scholars have discovered microplastics in salt, a finding that brought microplastics and humans closer together [ 77 ]. Subsequently, Karami et al. found microplastics in commercial salt from different countries, including Australia, France, Iran, Japan, Malaysia, New Zealand, and Portugal [ 78 ]. In summary, microplastics threaten freshwater organisms and food webs, and more understanding is needed to predict the future impact of microplastics.
5. Conclusions and Future Research
The main conclusions drawn from the visual analysis of publications, focal points, and international cooperation on microplastics in aquatic ecosystems are as follows:
- (1) Number of literature phases: Between 2013 and 2022, publications on microplastics went through three stages: budding development (2013–2015), slow rise (2016–2018), and rapid development (2019–2022). The number of publications increased over time.
- (2) Trends in focal points: The research focus shifted from the basic morphological characteristics, distribution, and fundamental impacts of microplastics (2013–2017) to the complex impacts of microplastics (2018–2022). In the earlier period (2013–2017), the effects of microplastics were primarily studied in terms of their shape and polymer type. In the later period (2018–2022), the focus expanded to include species, organisms, transport, toxicity, and other related factors.
- (3) Trends in international cooperation: International cooperation in microplastics research has increased over time, with strong representation from countries such as China, the USA, Australia, and Europe. Collaborations often occur between English-speaking countries or between English and Spanish/Portuguese-speaking countries, indicating that language barriers may limit broader international collaboration. There are still many countries and regions globally, particularly in Africa, the Middle East, and South America, that are not extensively involved in international cooperation on microplastics research.
Future research should include the following:
- (1) Strengthening the cross-analysis of microplastics with chemistry and toxicology to study their sorption-pollution effects with different pollutants. Currently, research on the sorption contamination of microplastics is not extensive enough. While there is a focus on sorption contamination with antibiotics, heavy metals, and other substances, the contamination effects and toxicological impacts of microplastics combined with microorganisms, bacteria, pesticides, new pollutants, and other substances have been less studied. Therefore, future studies should expand the classification of the sorption-pollution effects of microplastics for different contaminants.
- (2) Strengthening the long-term monitoring of microplastics to explore the actual pollution process over extended periods. Most current studies on the effects of microplastics are conducted in laboratory settings, where the microplastics retain their original qualities, shapes, and materials [ 79 ]. However, microplastics undergo changes in the environment over time. Currently, there is a lack of long-term monitoring data in the field of microplastics research, which can hinder scholars’ understanding of the actual contamination process of microplastics. Therefore, future research should focus on strengthening long-term monitoring studies to investigate the evolving relationship between microplastics and the environment.
Acknowledgments
We would like to express our gratitude to Sangar Khan for his valuable input and review of the language used in this manuscript. We would also like to thank Shaojing Jiang for revising the article.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics11060539/s1 , Table S1: Countries involved in microplastics-related research and the volume of their publications, 2013–2017; Table S2: Countries involved in microplastics-related research and the volume of their publications, 2018-2022.
Funding Statement
This study was supported financially by the National Natural Science Foundation of China (Nos. 52279068, 42001014) and Natural Science Foundation of Zhejiang Province (No. Q21D010016).
Author Contributions
Conceptualization, Y.W. (Yaochun Wang). and N.W.; methodology, G.L.; software, Y.W. (Yixia Wang); validation, H.M. and X.S.; formal analysis, Y.W. (Yaochun Wang) and N.W.; investigation, C.W.; resources, C.W.; data curation, X.S.; writing—original draft preparation, Y.W. (Yaochun Wang); writing—review and editing, Y.W. (Yaochun Wang); visualization, N.W.; supervision, N.W.; project administration, N.W.; funding acquisition, N.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Freshwater Ecosystems
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Last »
- Ecosystem Services Follow Following
- Adaptation Follow Following
- Resilience Follow Following
- Freshwater Ecology Follow Following
- Climate Change Follow Following
- Macroinvertebrates Follow Following
- Freshwater Biology Follow Following
- Recherche Follow Following
- Ecohydraulics Follow Following
- Freshwater ecology (Biology) (Biology) Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Journals
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Advertisement
Scientific literature on freshwater ecosystem services: trends, biases, and future directions
- AQUATIC ECOSYSTEM SERVICES
- Review Paper
- Published: 23 September 2022
- Volume 850 , pages 2485–2499, ( 2023 )
Cite this article

- João Carlos Nabout ORCID: orcid.org/0000-0001-9102-3627 1 ,
- Karine Borges Machado 1 ,
- Ana Clara Maciel David 1 ,
- Laura Beatriz Gomes Mendonça 2 ,
- Samiris Pereira da Silva 1 &
- Priscilla Carvalho 2
1116 Accesses
3 Citations
4 Altmetric
Explore all metrics
The aim of this study was to conduct a systematic review of the scientific literature on aquatic ecosystem services. We used the Web of Science database for our research. We found a total of 1,367 articles, with 837 papers that studied ecosystem services provided through the interaction between terrestrial and aquatic environments (terrestrial–aquatic papers) and 530 papers relating only to aquatic environments. Despite the growing number of articles, there is still less research on aquatic environments compared to terrestrial environments. We found a correlation between types of services and taxonomic groups, such that supporting ecosystem services were investigated using mainly macroinvertebrate, microorganisms and aquatic macrophytes communities, while provisioning and cultural ecosystem services were frequently studied with fishes. The analysis of keywords revealed that there was a temporal shift in the terms studied, and some words presented a higher frequency in aquatic research. Nonetheless, other words appeared in aquatic and terrestrial–aquatic studies with similar frequencies. The trends and biases detected in the present paper can help us to understand the role of aquatic biodiversity in ecosystem services more fully in the future as well as assist in the creation of strategies for future studies on the conservation of these services.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Artificial aquatic habitats: a systematic literature review and new perspectives

From ecological functions to ecosystem services: linking coastal lagoons biodiversity with human well-being
Use of ecosystem health indicators for assessing anthropogenic impacts on freshwaters in Argentina: a review
Data availability.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent to publication
Not applicable.
Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, et al., 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58(5): 403–414. https://doi.org/10.1641/B580507
Article Google Scholar
Acharya, R. P., T. Maraseni & G. Cockfield, 2019. Global trend of forest ecosystem services valuation – An analysis of publications. Ecosystem Services 39: 100979.
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2005. Conservation of the biodiversity of Brazil’s inland waters. Conservation Biology 19: 646–652.
Alencar, B. T. B., V. H. V. Ribeiro, C. M. Cabral, N. M. C. Santos, E. A. Ferreira, D. M. T. Francino, J. B. Santos, D. V. Silva & M. F. Souza, 2020. Use of macrophytes to reduce the contamination of water resources by Pesticides. Ecological Indicators 109: 105785. https://doi.org/10.1016/j.ecolind.2019.105785
Article CAS Google Scholar
Alexandrov, G. A., V. A. Brovkin, T. Kleinen & Y. Zicheng, 2020. The capacity of northern peatlands for long-term carbon sequestration. Biogeosciences 17: 47–54.
Allan, J. D., N. F. Manning, S. D. P. Smith, C. E. Dickinson, C. A. Joseph & D. R. Pearsall, 2017. Ecosystem services of Lake Erie: spatial distribution and concordance of multiple services. Journal of Great Lakes Research 43(4): 678–688.
Aznar-Sánchez, J. A., L. J. Belmonte-Ureña, M. J. López-Serrano & J. F. Velasco-Muñoz, 2018. Forest ecosystem services: an analysis of worldwide research. Forests 9(8): 453.
Aznar-Sánchez, J. A., J. F. Velasco-Muñoz, L. J. Belmonte-Ureña & F. Manzano-Agugliaro, 2019. The worldwide research trends on water ecosystem services. Ecological Indicators 99: 310–323.
Ballinger, A. & P. S. Lake, 2006. Energy and nutrient fluxes from rivers and streams into terrestrial food webs. Marine and Freshwater Research 57(1): 15–28.
Carneiro, F. M., J. C. Nabout & L. M. Bini, 2008. Trends in the scientific literature on phytoplankton. Limnology 9(2): 153–158.
Carvalho, R. M. & C. F. Szlafsztein, 2019. Urban vegetation loss and ecosystem services: the influence on climate regulation and noise and air pollution. Environmental Pollution 245: 844–852.
Article PubMed Google Scholar
Chan, K. M. A., T. Satterfield & J. Goldstein, 2012. Rethinking ecosystem services to better address and navigate cultural values. Ecological Economics 74: 8–18.
Chapman, R. L., 2013. Algae: the world’s most important “plants” – an introduction. Mitigation and Adaptation Strategies for Global Change 18: 5–12. https://doi.org/10.1007/s11027-010-9255-9
Cheng, B. & H. Li, 2020. Impact of climate change and human activities on economic values produced by ecosystem service functions of rivers in water shortage area of Northwest China. Environmental Science and Pollution Research 27: 26570–26578. https://doi.org/10.1007/s11356-020-08963-2
Clerici, N., M. L. Paracchini & J. Maes, 2014. Land-cover change dynamics and insights into ecosystem services in European stream riparian zones. Ecohydrology and Hydrobiology 14(2): 107–120.
Costanza, R., R. d’Arge, R. de Groot, S. Farber, M. Grasso, B. Hannon, K. Limburg, S. Naeem, R. V. O’Neill, J. Paruelo, et al., 1997. The value of the world’s ecosystem services and natural capital. Nature 387: 253–260.
Cotner, J. B. & B. A. Biddanda, 2002. Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5: 105–121.
Covich, A. P., K. C. Ewel, R. O. Hall Jr., P. S. Giller, W. Goedkoop & D. M. Merritt, 2004. Ecosystem services provided by freshwater benthos. In Wall, D. H. (ed), Sustaining Biodiversity and Functioning in Soils and Sediments Island Press, Washington, DC: 45–73.
Google Scholar
Dahlin, K. M., P. L. Zarnetske, Q. D. Read, L. A. Twardochleb, A. G. Kamoske, K. S. Cheruvelil & P. A. Soranno, 2021. Linking terrestrial and aquatic biodiversity to ecosystem function across scales, trophic levels, and realms. Frontiers in Environmental Science. https://doi.org/10.3389/fenvs.2021.692401 .
Danley, B. & C. Widmark, 2016. Evaluating conceptual definitions of ecosystem services and their implications. Ecological Economics 126: 132–138. https://doi.org/10.1016/j.ecolecon.2016.04.00
Dreyer, J., D. Hoekman & C. Gratton, 2011. Lake-derived midges increase abundance of shoreline terrestrial arthropods via multiple trophic pathways. Oikos 121(2): 252–258.
Dudgeon, D., 2019. Multiple threats imperil freshwater biodiversity in the Anthropocene. Current Biology 29: R960–R967.
Article CAS PubMed Google Scholar
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A. H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Engelhardt, K. A. & M. E. Ritchie, 2002. The effect of aquatic plant species richness on wetland ecosystem processes. Ecology 83: 2911–2924.
Englund, O., G. Berndes & C. Cederberg, 2017. How to analyse ecosystem services in landscapes – a systematic review. Ecological Indicators 73: 492–504.
Gangahagedara, R., S. Subasinghe, M. Lankathilake, W. Athukorala & I. Gamage, 2021. Ecosystem services research trends: a bibliometric analysis from 2000–2020. Ecologies 2: 366–379. https://doi.org/10.3390/ecologies2040021
Garau, E., J. Vila-Subiros, J. Pueyo-Ros & A. R. Palom, 2020. Where do ecosystem services come from? Assessing and mapping stakeholder perceptions on water ecosystem services in the Muga River Basin (Catalonia, Spain). Land 9(10): 385. https://doi.org/10.3390/land9100385
Ghosh, S., S. Chatterjee, G. S. Prasad & P. Pal, 2020. Effect of climate change on Aquatic ecosystem and production of fisheries. In Devlin, A. (ed), Inland Waters: Dynamics and Ecology. IntechOpen: 43–52. https://doi.org/10.5772/intechopen.93784 .
Gopal, B., 2016. Should ‘wetlands’ cover all aquatic ecosystems and do macrophytes make a difference to their ecosystem services? Folia Geobotanica 51: 209–226.
Hardin, G., 1968. The tragedy of the commons: the population problem has no technical solution; it requires a fundamental extension in morality. Science 162(3859): 1243–1248.
Haunschild, R., L. Bornmann & W. Marx, 2016. Climate change research in view of bibliometrics. PLoS ONE 11(7): e0160393. https://doi.org/10.1371/journal.pone.0160393
Article CAS PubMed PubMed Central Google Scholar
Holmlund, C. M. & M. Hammer, 1999. Ecosystem services generated by fish populations. Ecological Economics 29(2): 253–268. https://doi.org/10.1016/s0921-8009(99)00015-4
Hou, Y., S. Ding, W. Chen, B. Li, B. Burkhard, S. Bicking & F. Müller, 2020. Ecosystem service potential, flow, demand and their spatial associations: a comparison of the nutrient retention service between a human-and a nature-dominated watershed. Science of the Total Environment 748: 141341.
Jablońska, E., M. Winkowska, M. Wiśniewska, J. Geurts, D. Zak & W. Kotowski, 2021. Impact of vegetation harvesting on nutrient removal and plant biomass quality in wetland buffer zones. Hydrobiologia 848: 3273–3289.
Jenkins, W. A., B. C. Murray, R. A. Kramer & S. P. Faulkner, 2010. Valuing ecosystem services from wetlands restoration in the Mississippi Alluvial Valley. Ecological Economics 69(5): 1051–1061.
Josué, I. I. P., E. O. Sodré, R. B. Setubal, S. J. Cardoso, F. Roland, M. P. Figueiredo-Barros & R. L. Bozelli, 2021. Zooplankton functional diversity as an indicator of along-term aquatic restoration in an Amazonian lake. Restoration Ecology 29: e13365.
Joy, N. M. & S. K. Paul, 2020. Analysis of the economic value and status of the ecosystem services provided by the Ashtamudi Wetland Region, a Ramsar Site in Kerala. Journal of the Indian Society of Remote Sensing 49: 897–912. https://doi.org/10.1007/s12524-020-01263-9
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106(1): 110–127.
Kaiser-Bunbury, C. N., J. Mougal, A. E. Whittington, T. Valentin, R. Gabriel, J. M. Olesen & N. Blüthgen, 2017. Ecosystem restoration strengthens pollination network resilience and function. Nature 542: 223–227.
Keller, R. P., A. Masoodi & R. T. Shackleton, 2017. The impact of invasive aquatic plants on ecosystem services and human well-being in Wular Lake, India. Regional Environmental Change 18(3): 847–857.
Kensa, V. M., 2012. Climate change and its impacts on aquatic ecosystems. Journal of Industrial Pollution Control 28(1): 63–66.
Klein, A.-M., B. E. Vaissière, J. H. Cane, I. Steffan-Dewenter, S. A. Cunningham, C. Kremen & T. Tscharntke, 2007. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society b: Biological Sciences 274: 303–313.
Lê, S., J. Josse & F. Husson, 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25(1): 1–18. https://doi.org/10.18637/jss.v025.i01
Likens, G. E. & F. H. Bormann, 1974. Linkages between terrestrial and aquatic ecosystems. BioScience 24(8): 447-456.
Lima, F. P. & R. P. Bastos, 2020. Understanding landowners’ intention to restore native areas: the role of ecosystem services. Ecosystem Services 44: 101121. https://doi.org/10.1016/j.ecoser.2020.101121
Limburg, K. E., 2009. Aquatic ecosystem services. In Likens, G. E. (ed), Encyclopedia of Inland Waters Elsevier, Oxford: 25–30.
Chapter Google Scholar
Liquete, C., C. Piroddi, E. G. Drakou, L. Gurney, S. Katsanevakis, A. Charef & B. Egoh, 2013. Current status and future prospects for the assessment of marine and coastal ecosystem services: a systematic review. PLoS ONE 8(7): e67737.
Locke, D. H. & T. McPhearson, 2018. Urban areas do provide ecosystem services. Frontiers in Ecology and the Environment 16: 203–205.
Luck, G. W., G. C. Daily & P. R. Ehrlich, 2003. Population diversity and ecosystem services. Trends in Ecology and Evolution 18(7): 331–336. https://doi.org/10.1016/S0169-5347(03)00100-9
Mace, G. M., K. Norris & A. H. Fitter, 2012. Biodiversity and ecosystem services: a multilayered relationship. Trends in Ecology and Evolution 27: 19–26. https://doi.org/10.1016/j.tree.2011.08.006
Maseyk, F. J. F., A. D. Mackay, H. P. Possingham, E. J. Dominati & Y. M. Buckley, 2016. Managing natural capital stocks for the provision of ecosystem services. Conservation Letters 10(2): 211–220.
Matos, P., J. Vieira, B. Rocha, C. Branquinho & P. Pinho, 2019. Modeling the provision of air-quality regulation ecosystem service provided by urban green spaces using lichens as ecological indicators. Science of the Total Environment 665: 521–530. https://doi.org/10.1016/j.scitotenv.2019.02.023
McDonough, K., S. Hutchinson, T. Moore & J. M. S. Hutchinson, 2017. Analysis of publication trends in ecosystem services research. Ecosystem Services 25: 82–88.
Meyer, J. L., M. J. Sale, P. J. Mulholland & N. L. Poff, 1999. Impacts of climate change on aquatic ecosystem functioning and health. Journal of the American Water Resources Association 35(6): 1373–1386.
Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-Being: Synthesis, Island Press, Washington, DC:
Mitsch, W. J., B. Bernal & M. E. Hernandez, 2015. Ecosystem services of wetlands. International Journal of Biodiversity Science, Ecosystem Services and Management 11: 1–4.
Mittermeier, R. A., P. R. Gil & C. G. Mittermeier, 1997. Megadiversity, Earth’s Biologically Wealthiest Nations, CEMEX, Mexico City:
Monzó, J. C. & J. R. Verdú, 2022. Effects of restoration and management of Mediterranean traditional water systems on Odonata alpha diversity: a long-term monitoring survey. Biodiversity and Conservation 31: 227–243.
Nabout, J. C., P. Carvalho, M. U. Prado, P. P. Borges, K. B. Machado, K. B. Haddad & T. N. Soares, 2012. Trends and biases in global climate change literature. Natureza & Conservação 10(1): 45–51.
Nabout, J. C., G. Tessarolo, G. H. B. Pinheiro, L. A. M. Marquez & R. A. de Carvalho, 2022. Unraveling the paths of water as aquatic cultural services for the ecotourism in Brazilian Protected Areas. Global Ecology and Conservation 33: e01958.
Nakagawa, S., G. Samarasinghe, N. R. Haddaway, M. J. Westgate, R. E. O’Dea, D. W. Noble & M. Lagisz, 2019. Research weaving: visualizing the future of research synthesis. Trends in Ecology and Evolution 34(3): 224–238.
Naselli-Flores, L. & J. Padisák, 2022. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia. https://doi.org/10.1007/s10750-022-04795-y .
Article PubMed PubMed Central Google Scholar
Noriega, J. A., J. Hortal, F. M. Azcárate, M. P. Berg, N. Bonada, M. J. I. Briones & A. M. C. Santos, 2018. Research trends in ecosystem services provided by insects. Basic and Applied Ecology 26: 8–23.
Ondiek, R. A., N. Kitaka & S. O. Oduor, 2016. Assessment of provisioning and cultural ecosystem services in natural wetlands and rice fields in Kano Floodplain, Kenya. Ecosystem Services 21: 166–173.
Pagiola, S., K. von Ritter & J. Bishop, 2004. Assessing the Economic Value of Ecosystem Conservation. The World Bank Environment Department, Environment Department Paper No. 101.
Parron, L. M., E. C. C. Fidalgo, A. P. Luz, M. M. Campanha, A. P. D. Turetta, B. C. C. G. Pedreira & R. B. Prado, 2019. Research on ecosystem services in Brazil: a systematic review. Ambiente e Agua – an Interdisciplinary Journal of Applied Science 14(3): e2263. https://doi.org/10.4136/1980-993X
Pelicice, F. M., et al., 2021. Large-scale degradation of the Tocantins-Araguaia River Basin. Environmental Management 68(4): 445–452.
Peng, J., X. Wang, Y. Liu, Y. Zhao, Z. Xu, M. Zhao, et al., 2020. Urbanization impact on the supply–demand budget of ecosystem services: decoupling analysis. Ecosystem Services 44: 101139.
Porto, R. G., R. F. de Almeida, O. Cruz-Neto, M. Tabarelli, B. F. Viana, C. A. Peres & A. V. Lopes, 2020. Pollination ecosystem services: a comprehensive review of economic values, research funding and policy actions. Food Security 12: 1425–1442.
R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna [available on internet at http://www.R-project.org/ ].
Riis, T., et al., 2020. Global overview of ecosystem services provided by riparian vegetation. BioScience 70: 501–514.
Roth, B. M., I. C. Kaplan, G. G. Sass, P. T. Johnson, A. E. Marburg, A. C. Yannarell, T. D. Havlicek, T. V. Willis, M. G. Turner & S. R. Carpenter, 2007. Linking terrestrial and aquatic ecosystems: the role of woody habitat in lake food webs. Ecological Modelling 203: 439–452.
Roy, K., J. Vrba, S. J. Kaushik & J. Mraz, 2020. Nutrient footprint and ecosystem services of carp production in European fishponds in contrast to EU crop and livestock sectors. Journal of Cleaner Production 270: 122268.
Ruiz-Frau, A., S. Gelcich, I. E. Hendriks, C. M. Duarte & N. Marbà, 2017. Current state of seagrass ecosystem services: research and policy integration. Ocean and Coastal Management 149: 107–115.
Sica, Y., R. Quintana, V. Radeloff & G. Gavier-Pizarro, 2016. Wetland loss due to land use change in the Lower Paraná River Delta, Argentina. Science of the Total Environment 568: 967–978.
Silva, F. D. S., L. G. Carvalheiro, J. Aguirre-Gutiérrez, M. Lucotte, K. Guidoni-Martins & F. Mertens, 2021. Virtual pollination trade uncovers global dependence on biodiversity of developing countries. Science Advances 7: eabe6636. https://doi.org/10.1126/sciadv.abe6636
Stepniewska, M. & U. Sobczak, 2017. Assessing the synergies and trade-offs between ecosystem services provided by urban floodplains: the case of the Warta River Valley in Poznan, Poland. Land Use Police 69: 238–246.
Sudhakar, K., S. Suresh & M. Premalata, 2011. An overview of CO 2 mitigation using algae cultivation technology. International Journal of Chemical Research 3(3): 111–117.
Sweeney, B. W., T. L. Bott, J. K. Jackson, L. A. Kaplan, J. D. Newbold, L. J. Standley, W. C. Hession & R. J. Horwitz, 2004. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proceedings of the National Academy of Sciences of USA 101(39): 14132–14137.
Tang, S., L. C. Wang, X. G. Wu, Z. L. Xu & L. L. Chen, 2019. Dynamic evaluation and spatial mapping of wetland ecosystem services value – a case study on Nanjing Jiangbei new area. Environmental Engineering and Management Journal 18: 2519–2532.
TEEB, 2010. In Kumar, P. (ed), The Economics of Ecosystems and Biodiversity Ecological and Economic Foundations. Earthscan, London.
Thomaz, S. M., 2021. Ecosystem services provided by freshwater macrophytes. Hydrobiologia. https://doi.org/10.1007/s10750-021-04739-y .
Vallecillo, S., C. Polce, A. Barbosa, C. Perpiña Castillo, I. Vandecasteele, G. M. Rusch & J. Maes, 2018. Spatial alternatives for Green Infrastructure planning across the EU: an ecosystem service perspective. Landscape and Urban Planning 174: 41–54. https://doi.org/10.1016/j.landurbplan.2018.03.001
Vasconcelos, F. R., S. Diehl, P. Rodríguez, J. Karlsson & P. Byström, 2018. Effects of terrestrial organic matter on aquatic primary production as mediated by pelagic–benthic resource fluxes. Ecosystems 21: 1255–1268.
Vihervaara, P., M. Rönkä & M. Walls, 2010. Trends in ecosystem service research: early steps and current drivers. Ambio 39: 314–324.
Wang, C., C. Tang, B. Fu, Y. Lü, S. Xiao & J. Zhang, 2022. Determining critical thresholds of ecological restoration based on ecosystem service index: a case study in the Pingjiang catchment in southern China. Journal of Environmental Management 303: 114220.
Wickham, H., 2016. ggplot2: Elegant Graphics for Data Analysis. Springer, New York. ISBN 978-3-319-24277-4 [available on internet at https://ggplot2.tidyverse.org ].
Williams-Subiza, E. A. & L. B. Epele, 2021. Drivers of biodiversity loss in freshwater environments: a bibliometric analysis of the recent literature. Aquatic Conservation: Marine and Freshwater Ecosystems 31(9): 2469–2480.
Zedler, J. B. & S. Kercher, 2005. Wetland Resources: status, trends, ecosystem services, and restorability. Annual Review of Environment and Resources 30: 39–74.
Zhang, W., T. H. Ricketts, C. Kremen, K. Carney & S. M. Swinton, 2007. Ecosystem services and dis-services to agriculture. Ecological Economics 64(2): 253–260. https://doi.org/10.3390/su12239821
Zhang, X., R. C. E. Estoque, H. Xie, Y. Murayama & M. Ranagalage, 2019. Bibliometric analysis of highly cited articles on ecosystem services. PLoS ONE 14(2): e0210707.
Zhang, Y., R. Jin, W. Zhu, D. Zhang & X. Zhang, 2020. Impacts of land use changes on wetland ecosystem services in the Tumen River Basin. Sustainability 12(23): 9821. https://doi.org/10.3390/su12239821
Zhao, B. & Y. Su, 2020. Macro assessment of microalgae-based CO 2 sequestration: environmental and energy effects. Algal Research 51: 102066. https://doi.org/10.1016/j.algal.2020.102066
Zhou, T. & T. Endreny, 2020. The straightening of a River Meander leads to extensive losses in flow complexity and ecosystem services. Water 12(6): 1680. https://doi.org/10.3390/w12061680
Ziter, C., 2015. The biodiversity–ecosystem service relationship in urban areas: a quantitative review. Oikos 125(6): 761–768.
Download references
Acknowledgements
We are grateful to the funding agencies that provided scholarships and grants to the authors. JCN received Productivity Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). KBM received Post-doc Scholarship from Fundação de Amparo a Pesquisa do Estado de Goiás (FAPEG). ACMD received scholarship from FAPEG. LBGM received scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—code 001). SPS received scholarship from CNPq. Our work on Aquatic Ecology has been continuously supported by different grants: CNPq (Process 400870/2019-2), FAPEG (Process 201710267000519), and National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation (MCTI/CNPq/FAPEG/465610/2014-5), and Brazilian Network on Global Climate Change Research (Rede CLIMA).
FAPEG (Fundação de Amparo à Pesquisa do Estado de Goiás), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for scholarships and productivity grants. This study was financed in part by National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation (MCTI/CNPq/FAPEG/465610/2014-5), and Brazilian Network on Global Climate Change Research (Rede CLIMA).
Author information
Authors and affiliations.
Universidade Estadual de Goiás, Campus Central, Anápolis, GO, 75132-903, Brazil
João Carlos Nabout, Karine Borges Machado, Ana Clara Maciel David & Samiris Pereira da Silva
Universidade Federal de Goiás, Campus Samambaia, Goiânia, GO, 74690-900, Brazil
Laura Beatriz Gomes Mendonça & Priscilla Carvalho
You can also search for this author in PubMed Google Scholar
Contributions
JCN: Conception and design, analysis and interpretation of data, acquisition of data, and drafting of the article. KBM: Conception and design, analysis and interpretation of data, acquisition of data, drafting of the article and revising critically for important intellectual content. ACMD: Analysis and interpretation of data, acquisition of data and drafting of the article. LBGM: Analysis and interpretation of data, acquisition of data and drafting of the article. SPS: Analysis and interpretation of data, acquisition of data and drafting of the article. PC: Conception and design, analysis and interpretation of data, acquisition of data, drafting of the article, and revising critically for important intellectual content.
Corresponding author
Correspondence to João Carlos Nabout .
Ethics declarations
Conflict of interest.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
Not applicable. The present study did not involve research with humans or animals.
Informed consent
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Verónica Ferreira, Luis Mauricio Bini, Katya E. Kovalenko, Andre A. Padial, Judit Padisák & María de los Ángeles González Sagrario / Aquatic Ecosystem Services
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLSX 24 kb)
Supplementary file2 (docx 1376 kb), rights and permissions.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Nabout, J.C., Machado, K.B., David, A.C.M. et al. Scientific literature on freshwater ecosystem services: trends, biases, and future directions. Hydrobiologia 850 , 2485–2499 (2023). https://doi.org/10.1007/s10750-022-05012-6
Download citation
Received : 17 March 2022
Revised : 23 August 2022
Accepted : 02 September 2022
Published : 23 September 2022
Issue Date : July 2023
DOI : https://doi.org/10.1007/s10750-022-05012-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic review
- Supporting services
- Regulating services
- Provisioning services
- Cultural services
- Find a journal
- Publish with us
- Track your research

COMMENTS
The first paper, 'Integrating freshwater ecosystem health in water resources management' by Bogardi et al. deals with the policy framing for ensuring the good quality of waters and gives a valuable overview of a UN-based work, the UN water quality guidelines, with an extensive compilation of international background material.It assumes that deteriorating water quality also has an effect on ...
Freshwater ecosystems are among the most threatened in the world, while providing numerous essential ecosystem services (ES) to humans. Despite their importance, research on freshwater ecosystem services is limited. Here, we examine how freshwater studies could help to advance ES research and vice versa. We summarize major knowledge gaps and suggest solutions focusing on science and policy in ...
1. Introduction. Freshwater ecosystems provide a wide range of goods and ecosystem services for human well-being including provisioning of water supply for drinking, sanitation and irrigation (Green et al., 2015, Harrison et al., 2010).Besides, despite covering less than only 1% of the Earth's surface, freshwater ecosystems are remarkably rich and provide habitats for an about 10% of all ...
Freshwater Ecology. Contributions that investigate the ecology of freshwater ecosystems, including lakes, streams and rivers, wetlands, springs, bogs, and ponds. We are interested in a wide variety of papers - basic to applied, genes to biomes, microbes to mammals - that are conducted in freshwater and rooted in ecological principals and ...
A blueprint of freshwater life will: (i) build greater global awareness of the values of freshwater ecosystems and their species; (ii) mobilise the huge body of existing research information, such as on the functioning of wetlands, for application to the sustainable management and conservation of the world's freshwater ecosystems; (iii) fill ...
Journal of Freshwater Ecology is a fully open access journal for aquatic ecology of freshwater systems aimed at international researchers and professionals. The journal aims is to reflect the wide diversity of ecological subdisciplines and topics. Topics covered include, but are not limited to: Physiological; Population; Community; Ecosystem ...
Freshwater ecology is the study of all aspects of the ecology of terrestrial aquatic systems, including rivers, lakes and ponds. It includes the community ecology of these ecosystems, as well as ...
Freshwater ecosystems are among the most threatened in the world, while providing numerous essential ecosystem services (ES) to humans. Despite their importance, research on freshwater ecosystem services is limited. Here, we examine how freshwater studies could help to advance ES research and vice versa.
Metrics. Owing to a long history of anthropogenic pressures, freshwater ecosystems are among the most vulnerable to biodiversity loss 1. Mitigation measures, including wastewater treatment and ...
Freshwater ecosystems, particularly rivers and lakes, are under severe pressure due to increasing anthropogenic activities, such as water extraction, flow regulation, pollution, and habitat fragmentation [1,2,3].Local, regional, and global drivers of environmental change (e.g., land cover transformation, pollution, the introduction of invasive species, and climate change) are responsible for ...
Water biodiversity refers to the variety of life and ecosystems that make up freshwater, tidal, and marine regions of the world, as well as their interactions. Water biodiversity includes all freshwater ecosystems, including wetlands, lakes, ponds, reservoirs, rivers, streams, and groundwater (Jena & Gopalakrishnan, 2012). The marine ecosystems ...
Ecological monitoring and assessment is fundamental for effective management of ecosystems. As an introduction to this Special Issue, this editorial provides an overview of "Ecological Monitoring, Assessment, and Management in Freshwater Systems". This issue contains a review article on monitoring surface waters, and research papers on data management, biological assessment of aquatic ...
Submit your manuscript to Aquatic Conservation!. Aquatic Conservation: Marine and Freshwater Ecosystems is an international journal dedicated to the multidisciplinary science of conservation in all aquatic ecosystems - freshwater, brackish and marine. In doing so, we provide a nexus for discussion of aquatic conservation issues across the social, ecological, environmental and engineering ...
Water Environment Research is a multidisciplinary water and wastewater research journal, publishing fundamental and applied research related to water quality. Abstract This paper presents the reviews of scientific papers published in 2018 issues on the effects of anthropogenic pollution on the aquatic organisms dwelling in freshwater ecosystem ...
Abstract. Freshwater fish represent one-fourth of the world's vertebrates and provide irreplaceable goods and services but are increasingly affected by human activities. A new index, Cumulative Change in Biodiversity Facets, revealed marked changes in biodiversity in >50% of the world's rivers covering >40% of the world's continental ...
The purpose of this systematic review was to explore the most studied ecosystem services pro-vided by freshwater environments. We saw a trend of an increasing number of publications in recent years, which included all papers on ecosystem ser-vices, as well as those classified as aquatic and ter-restrial-aquatic papers.
Phytoplankton is a polyphyletic group with utmost. variation in size, shape, colour, type of metabolism, and life history traits. Due to the emerging knowledge. in nutritional capabilities of ...
Freshwater ecosystems are among the most threatened in the world, while providing numerous essential ecosystem services (ES) to humans. Despite their importance, research on freshwater ecosystem ...
The study of microplastics and their impact on aquatic ecosystems has received increasing attention in recent years. Drawing from an analysis of 814 papers related to microplastics published between 2013 and 2022 in the Web of Science Core Repository, this paper explores trends, focal points, and national collaborations in freshwater microplastics research, providing valuable insights for ...
In the ongoing global biodiversity crisis, freshwater habitats have been classified as one of the most vulnerable ecosystems (Dudgeon et al., 2006; WWF, 2020).In order to halt the decline in biodiversity and ecosystem functioning of these habitats, various legislative frameworks, such as the EU Water Framework Directive (WFD), the Clean Water Act of the USA (CWA) or the Water Act 2007 of ...
Crayfish as prime players in ecosystems. &amp;quot; Integrating research into freshwater biodiversity and the role of keystone species, this fascinating book presents freshwater crayfish as representatives of human-exacerbated threats to biodiversity and conservation. It... more. View Freshwater Ecosystems Research Papers on Academia.edu ...
Following these recommendations, Beijing's urban aquatic food web can be improved, leading to a healthier, more stable aquatic ecosystem. 5. Conclusion. Our research provides important insight into the increasing influence of variations in hydrodynamic (reduced flow velocity and increased water depth) and trophic conditions (reduced ...
Coastal upwelling supplies nutrients supporting primary production while also adding the toxic trace metal mercury (Hg) to the mixed layer of the ocean. This could be a concern for human and environmental health if it results in the enhanced bioaccumulation of monomethylmercury (MMHg). Here, we explore how upwelling influences Hg cycling in the California Current System (CCS) biome through ...
The aim of this study was to conduct a systematic review of the scientific literature on aquatic ecosystem services. We used the Web of Science database for our research. We found a total of 1,367 articles, with 837 papers that studied ecosystem services provided through the interaction between terrestrial and aquatic environments (terrestrial-aquatic papers) and 530 papers relating only to ...