Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.

Number of significant figures to put in a table?
Is there a well founded rule for the number of significant figures to publish?
Here are some specific examples / questions:
Is there any way to relate the number of significant figures to the coefficient of variation? For example, if the estimate is 12.3 and the CV is 50%, does that mean that the information represented by '.3' approaches zero?
If a confidence interval has a range of orders of magnitude, should they still have the same number of significant figures, e.g.:
12.3 (1.2, 123.4) vs 12 (1.2, 120)
Should the number of significant figures in an error estimate be the same or less than the number of significant figures in a mean?
- $\begingroup$ If you can, don't use a table :) A graphic is, IMO, almost always easier to read than a table (he obvious exception being if you don't have many numbers). Journals and their reviewers don't always agree, unfortunately.... $\endgroup$ – JMS Commented Mar 24, 2011 at 20:26
- 3 $\begingroup$ @JMS Good point, but Tables are useful for summarizing detailed characteristics of statistical units (cross-classified by a factor interest, e.g. clinical diagnosis or whatever), with variables of different types (continuous, nominal, and ordinal), and other results derived from statistical modeling per se (confusion matrix, regression coef. etc.) that won't fit into Figures (or not always if you think of Gelman's approach for showing reg. coef. as dotcharts). We need both; the question is when do we really need a Figure instead of a Table, IMO. $\endgroup$ – chl Commented Mar 24, 2011 at 22:37
- $\begingroup$ @chi Fair. I did say almost always :). Things like big n-way tables are impossible to (completely) reproduce graphically. It depends on the forum I'd say. Tables have the benefit of being complete, sure, but does your reader actually absorb all that extra information? If there are too many parameters to fit in a graph, I'd contend that a table is often at least difficult to read. However, I do think complete results should be accessible (online, appendix, etc) if for nothing else but reproducibility. In that case I'd also like data & code though! Wandered OT, sorry.. $\endgroup$ – JMS Commented Mar 25, 2011 at 3:57
- $\begingroup$ Also I think regression coefficients and confusion (correlation, covariance, ...) matrices are usually better suited to a graphical display, dotplots or similar for the former and heatmaps or graphs for the latter. $\endgroup$ – JMS Commented Mar 25, 2011 at 4:00
- $\begingroup$ @JMS I agree with your point, but in this case there is a figure limit, some other cases there are figure charges. Also, in this case if readers glance over the table and focus on the figures that are presented, then they won't waste time trying to figure out the point of an esoteric figure. But I fully support reproducibility, and while I am at it, I could (if I get around to it) add a visualization of the table to the code that is attached. $\endgroup$ – David LeBauer Commented Mar 25, 2011 at 5:40
2 Answers 2
I doubt there's a universal rule so I'm not going to make any up. I can share these thoughts and the reasons behind them:
When summaries reflect the data themselves--max, min, order statistics, etc.-- use the same number of significant figures used to record the data in the first place. This provides a consistent representation throughout the document concerning the precision of the data.
When summaries have higher precision than the data, write the values in a way that reflects that extra precision . For instance, a mean of $n$ values has $\sqrt{n}$ times the precision of the individual values: roughly, include one extra significant figure for $3 \le n \le 30$, two for $30 \lt n \le 300$, etc. (This is rounding on a log-10 scale, obviously.)
-Note that the CV does not provide useful information in this regard.
-Some estimates can be obtained with great precision. They don't have to be rounded to match something else. For instance, the mean of 1,000,000 integers might be 10.977 with a standard error of 0.00301. My decision to write the mean to three decimal places (and 4-5 sig figs) was based on the order of magnitude of the SE, which indicates the last digit is partially reliable. The decision to write the SE to three sig figs (five decimal places) is more arbitrary: two sig figs would work; one probably would not; four sig figs would also work and be consistent with the 4-5 sig figs in the mean; more than four sig figs would be overkill. (One could estimate the standard error of the SE itself in terms of the fourth moment of the data, and use that to determine an appropriate amount of rounding, but most of us don't go to such trouble...)
Signal the reader when you are doing substantial rounding . Be especially careful when the report is discussing the statistical test itself . The reason is that people may use your work to check their own calculations. Sometimes even a slight difference can reveal an error. You don't want to cause trouble because you rounded 123 to 120 and someone else, checking the work, obtains 123 and suspects one of you has erred.
Be consistent . You might lose some readers if you list a value as 123 at one point and later reference it as 120.
Don't be ridiculous . (I automatically suspect incompetence when I encounter reports that give statistical results to 15 sig figs when the data have only two sig figs, for instance.)
- 3 $\begingroup$ My very big +1 because it is really a lot of good advices. In the same vein, I like to show to students that it is really pointless to summarize data gathered from surveys (or votes) as % with a lot of decimals without considering sample size (which impacts standard error). $\endgroup$ – chl Commented Mar 24, 2011 at 22:46
I'd suggest 12 (1.2, 123.4). Omit the .3 since it's nearly meaningless, but many people when they see (1.2, 120) will assume that the last '0' in 120 is significant.
- $\begingroup$ Why do you suggest to omit a decimal for the statistic of interest if you agree to show them in the CIs (i.e., if it's meaningless for 12, why does it make sense for 123.4)? $\endgroup$ – chl Commented Mar 24, 2011 at 22:50
- $\begingroup$ @chl: it doesn't make much sense, but omitting it might be misleading. If I put in 123.4, someone like you will see the extra digits and just disregard them, no harm done. If I put in 120, many readers will think this is accurate to 3 digits - bad. $\endgroup$ – AVB Commented Mar 24, 2011 at 23:59
- $\begingroup$ still not clear why you recommend 123.4 instead of 123 (why omit .3 but not .4 in the example?) $\endgroup$ – David LeBauer Commented Mar 26, 2011 at 23:24
Your Answer
Sign up or log in, post as a guest.
Required, but never shown
By clicking “Post Your Answer”, you agree to our terms of service and acknowledge you have read our privacy policy .
Not the answer you're looking for? Browse other questions tagged tables or ask your own question .
- Featured on Meta
- We've made changes to our Terms of Service & Privacy Policy - July 2024
- Bringing clarity to status tag usage on meta sites
Hot Network Questions
- Including standalone tikz in beamer
- Why HIMEM was implemented as a DOS driver and not a TSR
- Making wobbly 6x4’ table stable
- Why are these typ. values greater than min. values in the datasheet of ADC128s102?
- What's wrong with my app authentication scheme?
- Rendering React SPAs within Salesforce
- If Venus had a sapient civilisation similar to our own prior to global resurfacing, would we know it?
- Is this equation of the GARCH(1,1) model correct?
- I submitted a paper and later realised one reference was missing, although I had written the authors in the body text. What could happen?
- How come I was allowed to bring 1.5 liters of liquid through security at Frankfurt Airport?
- Can a Statute of Limitations claim be rejected by the court?
- Does Gimp do batch processing?
- How did Jason Bourne know, the garbage man isn't CIA?
- Power line crossing data lines via the ground plane
- How predictable are the voting records of members of the US legislative branch?
- Prove that there's a consecutive sequence of days during which I took exactly 11 pills
- Finding the maximum squared distance between a pair of coordinates from a set of coordinates
- Why does editing '/etc/shells' file using 'sudo open' shows an error saying I don't own the file?
- How would a culture living in an extremely vertical environment deal with dead bodies?
- Which direction is the rolling shutter on a Canon 5D?
- How do *Trinitarians* explain why Jesus didn't mention the Holy Spirit in Matthew 24:36 regarding his return?
- Advice needed: Team needs developers, but company isn't posting jobs
- Is there any point "clean-installing" on a brand-new MacBook?
- Base change in Chriss-Ginzburg

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction
Rules for significant figures
Calculations with significant figures.

- How do Roman numerals work?
- Where do Roman numerals come from?
- Is it still important to learn Roman numerals?

significant figures
Our editors will review what you’ve submitted and determine whether to revise the article.
- Khan Academy - Intro to significant figures
- Indiana University - Meaningful Numbers and “Significant Figures”
- Open Library Publishing Platform - Significant Figures
- Open Washington Pressbooks - Significant Figures
- BCcampus Open Publishing - Significant Figures
- Chemistry LibreTexts - Significant Digits
- National Center for Biotechnology Information - PubMed Central - Significant Figures
- Table Of Contents

significant figures , any of the digits of a number beginning with the digit farthest to the left that is not zero and ending with the last digit farthest to the right that is either not zero or that is a zero but is considered to be exact. Significant figures are used to report a value , measured or calculated, to the correct number of decimal places or digits that will reflect the precision of the value.
The number of significant figures a value has depends on how it was measured, or how it was calculated. When a measurement is taken, the precision of that measurement is dependent on the equipment used to take the measurement. The measurement will have some digits that are certain and one digit that is uncertain, or estimated. The digits will be certain to the lowest increment division of the equipment used to take the measurement, and the estimated digit will be based on a best guess when the measurement is between two of the lowest increments of the equipment. If, for example, a measurement is taken with a metric ruler that has millimetre markings as the lowest increment, the number of millimetres of the measurement will be known for certain. One other digit will be estimated, since the measured quantity can be between two millimetre increments. If the item being measured falls exactly on a millimetre marking, then the estimated digit is written as a zero, to indicate that the digit of the measurement beyond the millimetre increments on the ruler is thought to be a zero, and not a nonzero estimated value.
The basic concept of significant figures in measurements relates to the fact that a measured value cannot be more precise than the equipment being used to make the measurement. It should be noted here that the equipment used to take a measurement does not determine the number of significant figures a measured value will have, only where the precision of a value must stop being written. Only one estimated value can ever be included in a measured quantity.
Determining the number of significant figures in measured quantities is essential when reporting the precision of measured values and the precision that can be reported when measured values are used in calculations. The rules for determining the number of significant figures are as follows:
- For example, the value 211.8 has four significant figures.
- Thus, the number 20,007, with three 0s between the 2 and 7, has a total of five significant figures.
- A value such as 0.0085, for example, has two significant figures because the 0s before the 8 are placeholders and are not significant.
- For example, a value written as 320. shows the decimal point, which indicates that the 0 to the right of the 2 was measured; therefore, the value has a total of three significant figures. If the decimal point was not written, then 320 would have only two significant figures. In general, any confusion this may cause can be avoided by writing values such as these in scientific notation.
- This means a value such as 12.000 has a total of five significant figures, since the 0s after the decimal place have been measured to be zeros, indicating they are as significant as any other nonzero digit.
- In a defining expression like 1 metre = 100 centimetres, these values are considered exact and thus have an infinite number of significant figures. While π is usually written as 3.14 for ease of calculation, the π button on the calculator would be used in any calculations, and thus it is considered to be a value with infinite significant figures.
- For example, the value 4,500 can be written in scientific notation to reflect two, three, and four significant digits:
- 4.5 × 10 3 has two significant figures
- 4.50 × 10 3 has three significant figures
- 4.500 × 10 3 has four significant figures
For calculations involving measured quantities, the first step in determining the precision of the answer is to determine the number of significant figures in each of the measured quantities. Once done, the number of significant figures in a calculated value involving measurements is determined based on the mathematical operation being performed.
When two or more measured quantities are added or subtracted, the resulting value will have the same number of decimal places as the value with the fewest number of decimal places (the limiting value). So if the measured values of 22.35 and 47.773 are added, the limiting value of 22.35 has two decimal places, which means that the result of the addition will have only two decimal places.
When two or more measured quantities are being multiplied or divided, the answer will have the same number of total significant figures as the value with the fewest number of significant figures. So if the measured values of 2.445 and 31.7 are being multiplied, the resulting value will have three significant figures, since 2.445 has four significant figures, but 31.7 has only three significant figures.
When a value is to be rounded off, the rules for rounding are:
- For example, 33.742 is to be rounded to one decimal place. Here, the 7 in the first decimal place is followed by a 4, which is less than 5, which means that 33.742 rounded to one decimal place is 33.7. Note that only the 4 that is to the right of the 7 is looked at here; the 2 in the third decimal place is insignificant when rounding to one decimal place.
- For example, 2.8763 is to be rounded to two decimal places. In this case, the 6 in the third decimal place is greater than 5, so the 7 in the second decimal place is rounded up to 8. This means that when rounded to two decimal places, 2.8763 rounds to 2.88. Again, the 3 in the fourth decimal place is insignificant when rounding to two decimal places.
- Thus, 21.45 rounds to one decimal place to 21.4, while 36.75 would round to 36.8.
- However, if a value such as 38.25003 is to be rounded to one decimal place, it rounds to 38.3. This is the only type of rounding where a digit farther than immediately to the right of the one being rounded to is ever considered. In this example, the digit looked at when rounding off to one decimal place is a 5. However, farther along the decimal portion of the value there is a nonzero digit. The number being rounded is therefore rounded up, as the 0.00003 indicates that the value of 0.05003 is larger than just the 0.05. For this reason, the value rounded to one decimal place is 38.3, not 38.2.
- Privacy Policy

Home » Figures in Research Paper – Examples and Guide
Figures in Research Paper – Examples and Guide
Table of Contents

Figures in Research Paper
Figures play an essential role in research papers as they provide a visual representation of data, results, and concepts presented in the text. Figures can include graphs, charts, diagrams, photographs, and other visual aids that enhance the reader’s understanding of the research.
Importance of Figures in Research Paper
Here are some specific ways in which figures can be important in a research paper:
- Visual representation of data : Figures can be used to present data in a clear and concise way. This makes it easier for readers to understand the results of experiments and studies.
- Simplify complex ideas: Some concepts can be difficult to explain using words alone. Figures can be used to simplify complex ideas and make them more accessible to a wider audience.
- Increase reader engagement : Figures can make a research paper more engaging and interesting to read. They break up long blocks of text and can make the paper more visually appealing.
- Support arguments: Figures can be used to support arguments made in the paper. For example, a graph or chart can be used to show a correlation between two variables, providing evidence for a particular hypothesis.
- Convey important information: Figures can be used to convey important information quickly and efficiently. This is particularly useful when the paper is being read by someone who is short on time and needs to quickly understand the main points.
Types of Figures in Research Paper
There are several types of figures commonly used in research papers, including:
- Line graphs: These are used to show trends or changes in data over time.
- Bar graphs: These are used to compare data across different categories or groups.
- Pie charts: These are used to show proportions or percentages of data.
- Scatterplots : These are used to show the relationship between two variables.
- Tables : These are used to present large amounts of data in a structured format.
- Photographs or images : These are used to provide visual context or examples of the research being presented.
- Diagrams or schematics : These are used to illustrate complex processes or systems.
How to add Figures to Research Paper
Adding figures to a research paper can be a great way to visually convey important information to the reader. Here are some general guidelines for adding figures to your research paper:
- Determine the appropriate type of figure: Depending on the information you want to convey, you may want to use a graph, chart, table, photograph, or other type of figure.
- Label the figure: Give your figure a descriptive title and number it. Also, include a brief caption that explains what the figure shows.
- Place the figure in the appropriate location : Generally, figures should be placed as close as possible to the text that refers to them. For example, if you mention a figure in the middle of a paragraph, it should be placed within that paragraph.
- Format the figure appropriately: Ensure that the figure is clear and easy to read. Use consistent fonts and font sizes, and make sure the figure is large enough to be easily seen.
- Cite the source of the figure: If the figure was not created by you, you must cite the source of the figure in your paper. This includes citing the author or creator, the date of creation, and any relevant publication information.
- Consider copyright : Ensure that you have permission to use any figures that are copyrighted. If the figure is copyrighted, you may need to obtain permission from the copyright holder to use it in your paper.
How to Label Figures in Research Paper
Labeling figures in a research paper is an important task that helps readers to understand the content of the paper. Here are the steps to label figures in a research paper:
- Decide on the numbering system: Before labeling the figures, decide on the numbering system that you want to use. Typically, figures are numbered consecutively throughout the paper, with the first figure being labeled as “Figure 1,” the second figure as “Figure 2,” and so on.
- Choose a clear and concise caption: A caption is a brief description of the figure that appears below the figure. It should be clear and concise and should describe the content of the figure accurately. The caption should be written in a way that readers can understand the figure without having to read the entire paper.
- Place the label and caption appropriately: The label and caption should be placed below the figure. The label should be centered and should include the figure number and a brief title. The caption should be placed below the label and should describe the figure in detail.
- Use consistent formatting: Make sure that the formatting of the labels and captions is consistent throughout the paper. Use the same font, size, and style for all figures in the paper.
- Reference figures in the text : When referring to a figure in the text, use the figure number and label. For example, “As shown in Figure 1, the results indicate that…”
Figure 1. Distribution of survey responses
In this example, “Figure 1” is the figure number, and “Distribution of survey responses” is a brief title or description of the figure.
The label should be placed at the top of the figure and should be centered. It should be clear and easy to read. It’s important to use a consistent format for all figures in the paper to make it easier for readers to follow.
Examples of Figures in Research Paper
Examples of Figures in Research Papers or Thesis are as follows:
Line graphs Example

Bar graphs Example

Pie charts Example

Scatterplots Example

Tables Example

Photographs or images Example

Diagrams or schematics Example

Purpose of Figures in Research Paper
Some common purposes of figures in research papers are:
- To summarize data: Figures can be used to present data in a concise and easy-to-understand manner. For example, graphs can be used to show trends or patterns in data, while tables can be used to summarize numerical information.
- To support arguments : Figures can be used to support arguments made in the text of the research paper. For example, a figure showing the results of an experiment can help to demonstrate the validity of the conclusions drawn from the experiment.
- To illustrate concepts: Figures can be used to illustrate abstract or complex concepts that are difficult to explain in words. For example, diagrams or illustrations can be used to show the structure of a complex molecule or the workings of a machine.
- To enhance readability: Figures can make a research paper more engaging and easier to read. By breaking up long blocks of text, figures can help to make the paper more visually appealing and easier to understand.
- To provide context : Figures can be used to provide context for the research being presented. For example, a map or diagram can help to show the location or layout of a study site or experimental setup.
- To compare results : Figures can be used to compare results from different experiments or studies. This can help to highlight similarities or differences in the data and draw comparisons between different research findings.
- To show relationships : Figures can be used to show relationships between different variables or factors. For example, a scatter plot can be used to show the correlation between two variables, while a network diagram can be used to show how different elements are connected to each other.
- To present raw data: Figures can be used to present raw data in a way that is easier to understand. For example, a heat map can be used to show the distribution of data over a geographic region, while a histogram can be used to show the distribution of data within a single variable.
Advantages of Figures in Research Paper
Figures (such as charts, graphs, diagrams, and photographs) are an important component of research papers and offer several advantages, including:
- Enhancing clarity : Figures can help to visually communicate complex data or information in a clear and concise manner. They can help readers better understand the research and its findings.
- Saving space : Figures can often convey information more efficiently than text, allowing researchers to present more information in less space.
- Improving readability : Figures can break up large blocks of text and make a paper more visually appealing and easier to read.
- Supporting arguments: Figures can be used to support arguments made in the text and help to strengthen the overall message of the paper.
- Enabling comparisons: Figures can be used to compare different data points, which can be difficult to do with text alone. This can help readers to see patterns and relationships in the data more easily.
- Providing context : Figures can provide context for the research, such as showing the geographic location of study sites or providing a visual representation of the study population.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Paper Format – Types, Examples and...

Assignment – Types, Examples and Writing Guide

Problem Statement – Writing Guide, Examples and...

Thesis Statement – Examples, Writing Guide

How to Cite Research Paper – All Formats and...

Research Paper Conclusion – Writing Guide and...
Get in touch
555-555-5555

Limited time offer: 20% off all templates ➞

How to Make Good Figures for Scientific Papers
Creating good figures for scientific publications requires using design best practices to make each figure clearly show the main point of your data story.
This article reviews important design principles that will help you create effective figures. However, if you want step-by-step tutorials on how to create the scientific illustrations and Excel graphs using Adobe Illustrator and PowerPoint, read these articles instead:
- Free Graphical Abstract Templates and Tutorials
- Free Research Poster Templates and Tutorials

Four Rules to Create High-Quality Figures
The best data visualizations for scientific papers use a combination of good design principles and storytelling that allows the audience to quickly understand the results of a scientific study. Below are four rules that will help you make effective research figures and save you time with the final journal formatting. There are also practical tips on how to find the purpose of your figure and how to apply design best practices to graphs, images, and tables.
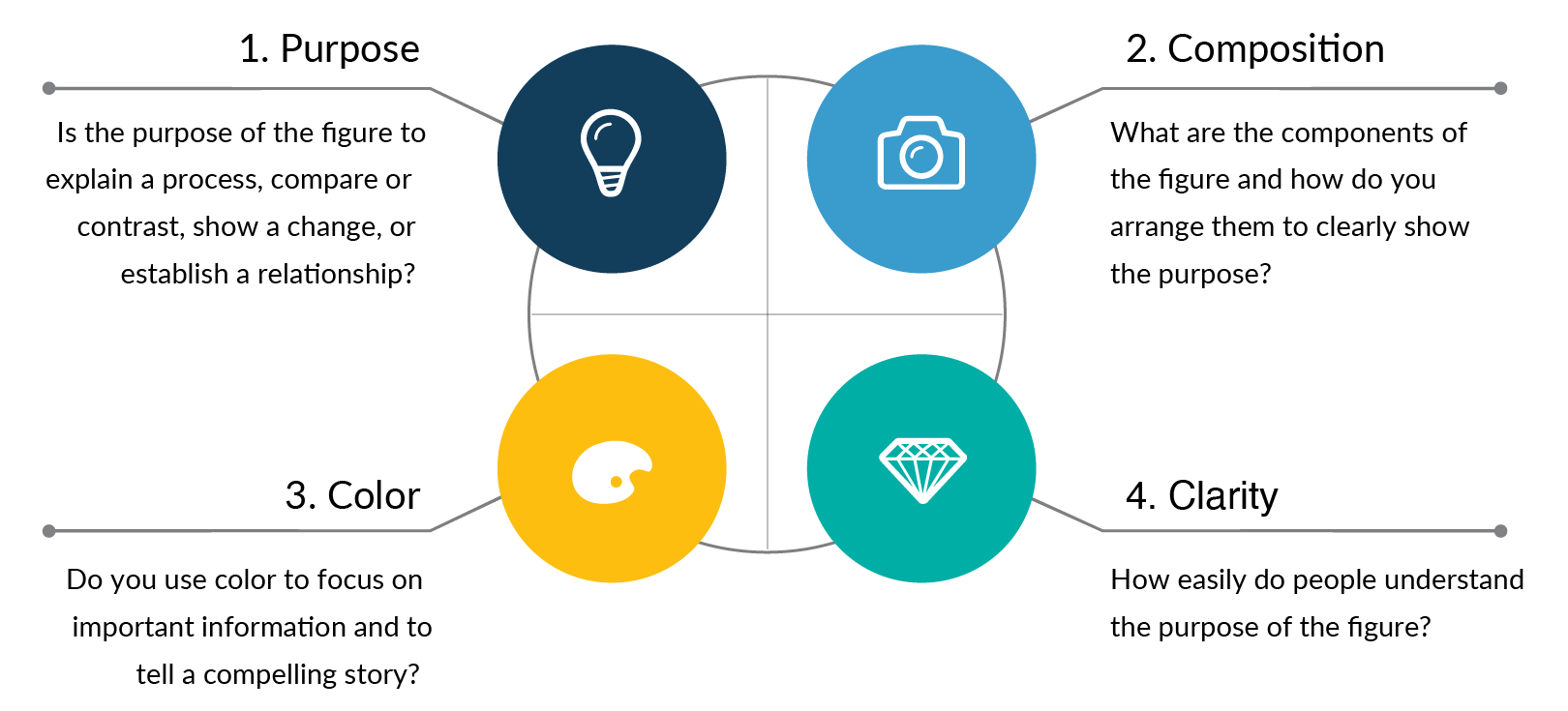
Rule 1: Clearly show the main purpose to your audience
For every graph or figure you create, the first step is to answer the question: what is the purpose of my data? Clearly defining the main purpose of your scientific design is essential so that you can create and format the data in ways that are easy to understand.
The most common purposes for scientific publications are to explain a process or method, compare or contrast, show a change, or to establish a relationship. Each of these purposes should then lead you to select graph types. For example, if the goal of your figure is to explain a method, you will likely want to choose process-focused graph types such as flow charts, diagrams, infographics, illustrations, gantt charts, timelines, parallel sets, or Sankey diagrams. Below are examples of the most common graph types that you can use for different data purposes. Read more articles to learn how to choose the right data visualizations and data storytelling .

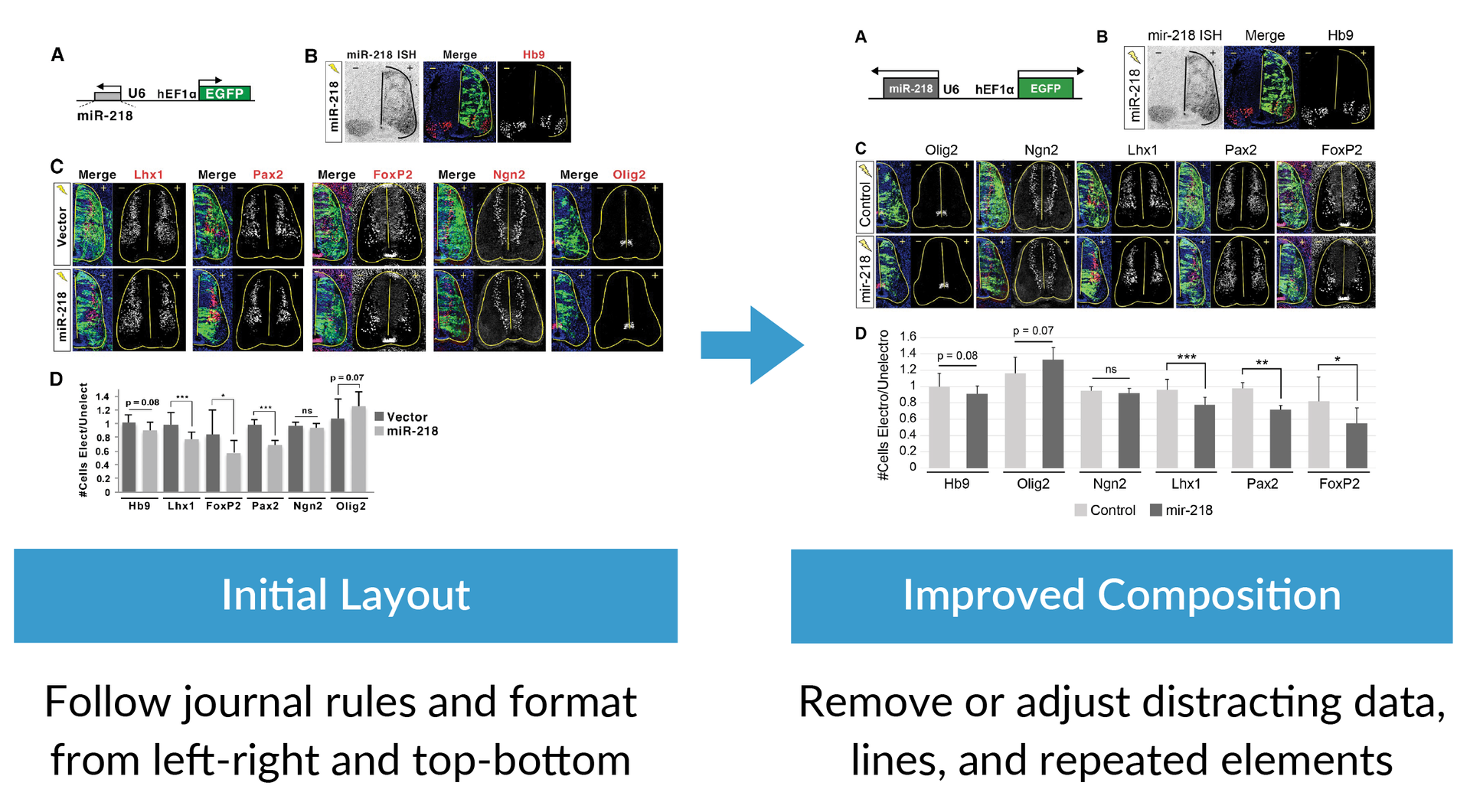
Rule 2: Use composition to simplify the information
After you define the purpose of your graph or figure, the next step is to make sure you follow composition best practices that make the information clear. Composition best practices include following the journal rules and formatting from left to right, top to bottom, or in a circle. You should also review your designs to remove or adjust distracting data, lines, shadows, and repeated elements. Applying good composition means spending time reviewing your layout and simplifying the story using these techniques.
Data Composition Best Practices:
- Design flow should be left to right, top to bottom, or in a circle
- Make sure most important data is the focus of the design
- Remove or adjust excess data and text
- Make text easy to read
- Reduce contrast of bold lines
- Remove repeated elements
- Remove shadows

The example below shows how to design a figure that applies the composition best practices by taking an initial layout of a figure on the left and then use formatting to fill the space, simplify information, and reorder the data to more clearly show the main purpose of the research.
Follow Science Journal Formatting Requirements:
In order to organize the graphs, charts, and figures, you will also need to know the requirements of the scientific journal. You will need to know the limits of the figure sizes, the maximum number of figures, as well as color, fonts, resolution, and file type requirements. You can find different journal requirements by going to the Journal’s homepage and then finding the link to the author’s guidelines from there. If you Google the journal’s formatting requirements, make sure you find the most up-to-date page.

For example, the academic journal Science allows a maximum of 6 figures and requires that they have a width of 55 mm (single column) or 230 mm (double column). In contrast, the journal Nature only allows 3-4 figures or tables with maximum widths of 89 mm (single column) and 183 mm (double column). If you planned to submit your scientific publication to Nature, you would need to carefully plan which graphs and tables will best tell your scientific story within only four figures.
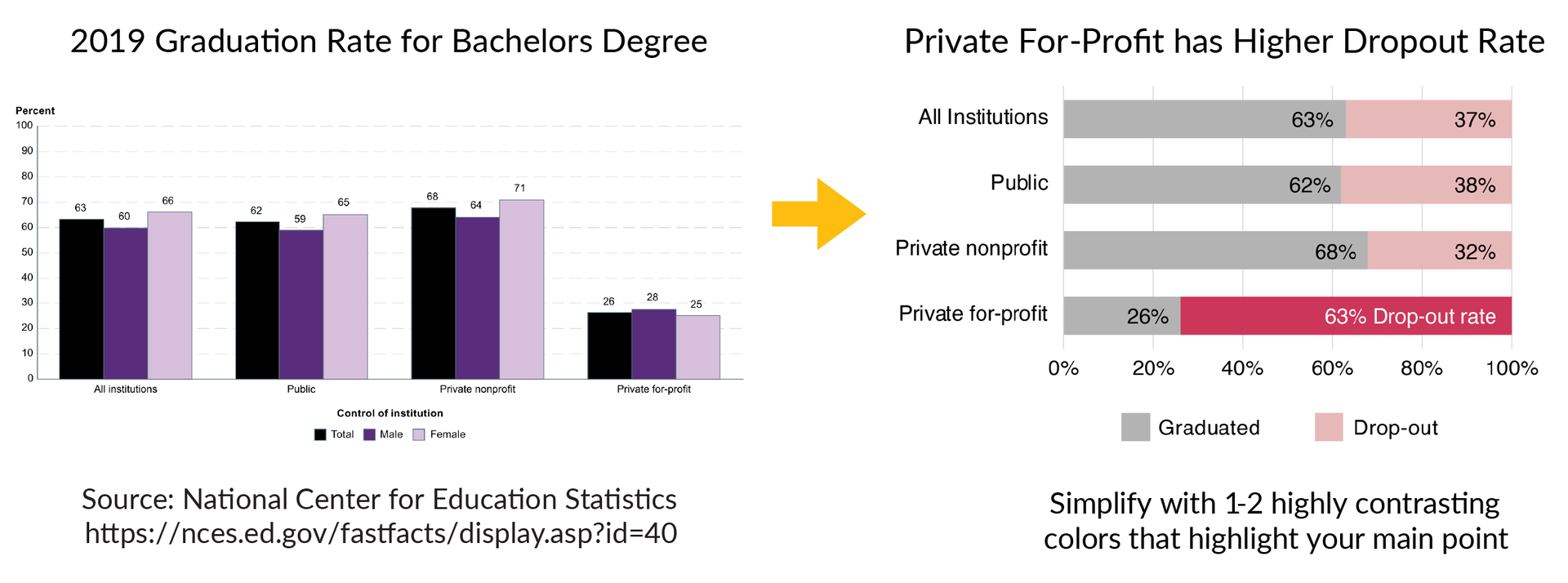
Rule 3: Use colors or grayscale to highlight the purpose
Color is one of the most powerful data storytelling tools. When used properly, color enhances understanding of your graphs and when used poorly, it can be very distracting.
Scientific Color Design Tips:
- If possible, limit your design to 1-2 colors that make the main point of the data stand out from the rest
- Make colors accessible to people with color blindness

The example below shows a graph on the left that has a lot of information about graduation rates for bachelor’s degrees in 2019. The text is small and the color design makes it difficult to understand the main results of the data. One way to improve this figure is to use colors to highlight the main story of the data, which is that private for-profit institutions have a much higher drop-out rate than all other institutions. The figure on the right improves this design using the bold pink color and clearer text to highlight the main point of the dataset.
Rule 4: Refine and repeat until the story is clear
The goal of good figure design is to have your audience clearly understand the main point of your research. That is why the final rule is to spend time refining the figure using the purpose, composition, and color tools so that the final design is clear.
It is normal to make 2-3 versions of a figure before you settle on the final design that works best. I recommend using the three clarity checkpoints below to improve your refinement process.

Design Clarity Checkpoints:
- Checkpoint 1. Does the figure show the overall story or main point when you hide the text? If not, improve the data visualization designs to more clearly show the main purpose.
- Checkpoint 2. Can you remove or adjust unnecessary elements that attract your attention? Remove repetitive elements, bounding boxes, background colors, extra lines, extra colors, repeated text, shadows/shading, either remove or adjust excess data, and consider moving information to supplementary figures.
- Checkpoint 3. Does the color palette enhance or distract from the story? Limit the use of color and pick a color palette that improves audience understanding of the main purpose of the figure. If the color doesn’t serve an obvious purpose, change to grayscale.
Scientific Figure Design Summary
For every scientific publication, follow the four rules of good scientific figure design to help you create effective graphics that engage and impress your audience:
- Clearly show the main purpose to your audience
- Use composition to simplify the information
- Use colors or grayscale to highlight the main points of the figure
- Refine and repeat the process until the story is clear
Related Content:
- Best Color Palettes for Scientific Figures and Data Visualizations
- Graphical Abstract Examples with Free Templates
- Free Research Poster Templates and Tutorials
- BioRender Alternatives: Scientific Illustration Software Comparisons
Create professional science figures with illustration services or use the online courses and templates to quickly learn how to make your own designs.
Interested in free design templates and training.
Explore scientific illustration templates and courses by creating a Simplified Science Publishing Log In. Whether you are new to data visualization design or have some experience, these resources will improve your ability to use both basic and advanced design tools.
Interested in reading more articles on scientific design? Learn more below:
Scientific Presentation Guide: How to Create an Engaging Research Talk

Data Storytelling Techniques: How to Tell a Great Data Story in 4 Steps

Best Science PowerPoint Templates and Slide Design Examples
Content is protected by Copyright license. Website visitors are welcome to share images and articles, however they must include the Simplified Science Publishing URL source link when shared. Thank you!
Online Courses
Stay up-to-date for new simplified science courses, subscribe to our newsletter.
Thank you for signing up!
You have been added to the emailing list and will only recieve updates when there are new courses or templates added to the website.
We use cookies on this site to enhance your user experience and we do not sell data. By using this website, you are giving your consent for us to set cookies: View Privacy Policy
Simplified Science Publishing, LLC

Sometimes you see the advice to round up figures and present them with the same number of decimals. However, this is not a good advice because it does not take into account that figures need to be presented with a varying number of decimals to reflect the underlying precision of the figures. Using the same number of decimals will present some figures less precise than they are while others are presented as being more precise than they are. We have two types of figures to consider in a research project; raw data and calculated data.
Table of Contents (with links)
Presenting your own results
Significant figures when presenting raw data.
If an observation is exact such as number of participants or number of children in a family then the exact number should be given. However, a lot of retained raw data are measurements producing a figure that is not exact. It is an estimate. Example can be height, weight, cholesterol levels in blood etc. Figures that are estimates should be given with a precision that reflects the accuracy of the measurement. Raw data can be presented with many significant figures if you are measuring using a method with very high accuracy. However, you should present the raw data with few significant figures if your method of measuring / estimating is unreliable . This is elegantly explained in the (rather funny) video “Why are Significant Figures Important?” by Tyler DeWitt:

Significant figures when presenting calculated data
Significant figures are also of interest as soon as you present any type of descriptive statistics or inferential statistics . How do I know what number of significant figures are appropriate? The number of significant figures that should be used when presenting research results (such as mean, standard deviation, odds ratios, p-values, etc) is given by the size of the sample you have. As a rule of thumb the appropriate number of significant figures can be obtained by taking the base 10 logarithm of the sample size and rounding to the nearest integer. The base 10 logarithm for a sample size of 100 is 2, for 1,000 is 3, for 10,000 is 4, for 100,000 is 5 and so forth.
Please note that if your raw data are very unreliable and only valid with one significant figure (unusual) it means that your calculated output should also be presented with only one significant figure. However, a lot of significant figures will make your manuscript more difficult to read and in most situations there is no need to present more than 3 significant figures even if your sample size is large enough to allow more significant figures. Some examples of proper rounding of figures:
| Result of a calculation / analysis | Two significant figures | Three significant figures |
|---|---|---|
| 148.6772 | 150 | 149 |
| 14.86335 | 15 | 14.9 |
| 1.35756 | 1.4 | 1.36 |
| 0.0653442 | 0.065 | 0.0653 |
| 0.0000021463 | 2.1 x 10 | 2.15 x 10 |
Consequences when writing a manuscript
The above means that you should vary the number of decimals when writing a manuscript. Please see the following examples.
- Percentages presented in table 1 in Nordeman et al 2017 and in table 1 in Tenenbaum et al 2017 .
- P-values presented in table 4 in Tenenbaum et al 2017 .
- Point estimate and confidence intervals for odds ratio as well as p-values in table 3 in Sundvall et al 2014 .
Systematic literature reviews
A systematic literature review does not aim to simply copy conclusions from previous authors. It aim to evaluate previous studies and if possible making new conclusions. It is common that authors of included studies use many more significant figures than their observations support. In this case don’t repeat their mistake. Reduce the number of significant figures to reflect what their observations actually support if previous authors of included publications provide more significant figures than their observations support.
However, don’t invent more significant figures (unless you have access to raw data so you can calculate yourself), if observations in previous publications support more significant figures than they provide. In many situations there is no reason to provide more than three significant figures even if the number of observations or precision of measurements would support that.
More about proper rounding of figures
Further details about how to round figures is explained in the video “How to Count and Round Significant Figures” by MahanChem:
Comments are closed.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
An Easy Introduction to Statistical Significance (With Examples)
Published on January 7, 2021 by Pritha Bhandari . Revised on June 22, 2023.
If a result is statistically significant , that means it’s unlikely to be explained solely by chance or random factors. In other words, a statistically significant result has a very low chance of occurring if there were no true effect in a research study.
The p value , or probability value, tells you the statistical significance of a finding. In most studies, a p value of 0.05 or less is considered statistically significant, but this threshold can also be set higher or lower.
Table of contents
How do you test for statistical significance, what is a significance level, problems with relying on statistical significance, other types of significance in research, other interesting articles, frequently asked questions about statistical significance.
In quantitative research , data are analyzed through null hypothesis significance testing, or hypothesis testing. This is a formal procedure for assessing whether a relationship between variables or a difference between groups is statistically significant.
Null and alternative hypotheses
To begin, research predictions are rephrased into two main hypotheses: the null and alternative hypothesis .
- A null hypothesis ( H 0 ) always predicts no true effect, no relationship between variables , or no difference between groups.
- An alternative hypothesis ( H a or H 1 ) states your main prediction of a true effect, a relationship between variables, or a difference between groups.
Hypothesis testin g always starts with the assumption that the null hypothesis is true. Using this procedure, you can assess the likelihood (probability) of obtaining your results under this assumption. Based on the outcome of the test, you can reject or retain the null hypothesis.
- H 0 : There is no difference in happiness between actively smiling and not smiling.
- H a : Actively smiling leads to more happiness than not smiling.
Test statistics and p values
Every statistical test produces:
- A test statistic that indicates how closely your data match the null hypothesis.
- A corresponding p value that tells you the probability of obtaining this result if the null hypothesis is true.
The p value determines statistical significance. An extremely low p value indicates high statistical significance, while a high p value means low or no statistical significance.
Next, you perform a t test to see whether actively smiling leads to more happiness. Using the difference in average happiness between the two groups, you calculate:
- a t value (the test statistic) that tells you how much the sample data differs from the null hypothesis,
- a p value showing the likelihood of finding this result if the null hypothesis is true.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
The significance level , or alpha (α), is a value that the researcher sets in advance as the threshold for statistical significance. It is the maximum risk of making a false positive conclusion ( Type I error ) that you are willing to accept .
In a hypothesis test, the p value is compared to the significance level to decide whether to reject the null hypothesis.
- If the p value is higher than the significance level, the null hypothesis is not refuted, and the results are not statistically significant .
- If the p value is lower than the significance level, the results are interpreted as refuting the null hypothesis and reported as statistically significant .
Usually, the significance level is set to 0.05 or 5%. That means your results must have a 5% or lower chance of occurring under the null hypothesis to be considered statistically significant.
The significance level can be lowered for a more conservative test. That means an effect has to be larger to be considered statistically significant.
The significance level may also be set higher for significance testing in non-academic marketing or business contexts. This makes the study less rigorous and increases the probability of finding a statistically significant result.
As best practice, you should set a significance level before you begin your study. Otherwise, you can easily manipulate your results to match your research predictions.
It’s important to note that hypothesis testing can only show you whether or not to reject the null hypothesis in favor of the alternative hypothesis. It can never “prove” the null hypothesis, because the lack of a statistically significant effect doesn’t mean that absolutely no effect exists.
When reporting statistical significance, include relevant descriptive statistics about your data (e.g., means and standard deviations ) as well as the test statistic and p value.
There are various critiques of the concept of statistical significance and how it is used in research.
Researchers classify results as statistically significant or non-significant using a conventional threshold that lacks any theoretical or practical basis. This means that even a tiny 0.001 decrease in a p value can convert a research finding from statistically non-significant to significant with almost no real change in the effect.
On its own, statistical significance may also be misleading because it’s affected by sample size. In extremely large samples , you’re more likely to obtain statistically significant results, even if the effect is actually small or negligible in the real world. This means that small effects are often exaggerated if they meet the significance threshold, while interesting results are ignored when they fall short of meeting the threshold.
The strong emphasis on statistical significance has led to a serious publication bias and replication crisis in the social sciences and medicine over the last few decades. Results are usually only published in academic journals if they show statistically significant results—but statistically significant results often can’t be reproduced in high quality replication studies.
As a result, many scientists call for retiring statistical significance as a decision-making tool in favor of more nuanced approaches to interpreting results.
That’s why APA guidelines advise reporting not only p values but also effect sizes and confidence intervals wherever possible to show the real world implications of a research outcome.
Aside from statistical significance, clinical significance and practical significance are also important research outcomes.
Practical significance shows you whether the research outcome is important enough to be meaningful in the real world. It’s indicated by the effect size of the study.
Clinical significance is relevant for intervention and treatment studies. A treatment is considered clinically significant when it tangibly or substantially improves the lives of patients.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Descriptive statistics
- Measures of central tendency
- Correlation coefficient
Methodology
- Cluster sampling
- Stratified sampling
- Types of interviews
- Cohort study
- Thematic analysis
Research bias
- Implicit bias
- Cognitive bias
- Survivorship bias
- Availability heuristic
- Nonresponse bias
- Regression to the mean
Statistical significance is a term used by researchers to state that it is unlikely their observations could have occurred under the null hypothesis of a statistical test . Significance is usually denoted by a p -value , or probability value.
Statistical significance is arbitrary – it depends on the threshold, or alpha value, chosen by the researcher. The most common threshold is p < 0.05, which means that the data is likely to occur less than 5% of the time under the null hypothesis .
When the p -value falls below the chosen alpha value, then we say the result of the test is statistically significant.
A p -value , or probability value, is a number describing how likely it is that your data would have occurred under the null hypothesis of your statistical test .
P -values are usually automatically calculated by the program you use to perform your statistical test. They can also be estimated using p -value tables for the relevant test statistic .
P -values are calculated from the null distribution of the test statistic. They tell you how often a test statistic is expected to occur under the null hypothesis of the statistical test, based on where it falls in the null distribution.
If the test statistic is far from the mean of the null distribution, then the p -value will be small, showing that the test statistic is not likely to have occurred under the null hypothesis.
No. The p -value only tells you how likely the data you have observed is to have occurred under the null hypothesis .
If the p -value is below your threshold of significance (typically p < 0.05), then you can reject the null hypothesis, but this does not necessarily mean that your alternative hypothesis is true.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bhandari, P. (2023, June 22). An Easy Introduction to Statistical Significance (With Examples). Scribbr. Retrieved August 12, 2024, from https://www.scribbr.com/statistics/statistical-significance/
Is this article helpful?

Pritha Bhandari
Other students also liked, understanding p values | definition and examples, what is effect size and why does it matter (examples), hypothesis testing | a step-by-step guide with easy examples, what is your plagiarism score.
Significant Figures
It is important to be honest when reporting a measurement, so that it does not appear to be more accurate than the equipment used to make the measurement allows. We can achieve this by controlling the number of digits, or significant figures , used to report the measurement.
Determining the Number of Significant Figures
The number of significant figures in a measurement, such as 2.531, is equal to the number of digits that are known with some degree of confidence (2, 5, and 3) plus the last digit (1), which is an estimate or approximation. As we improve the sensitivity of the equipment used to make a measurement, the number of significant figures increases.
| Postage Scale | 3 ±1 g | 1 significant figure | ||
| Two-pan balance | 2.53 ±0.01 g | 3 significant figures | ||
| Analytical balance | 2.531 ±0.001 g | 4 significant figures |
Rules for counting significant figures are summarized below.
Zeros within a number are always significant. Both 4308 and 40.05 contain four significant figures.
Zeros that do nothing but set the decimal point are not significant. Thus, 470,000 has two significant figures.
Trailing zeros that aren't needed to hold the decimal point are significant. For example, 4.00 has three significant figures.
If you are not sure whether a digit is significant, assume that it isn't. For example, if the directions for an experiment read: "Add the sample to 400 mL of water," assume the volume of water is known to one significant figure.
Addition and Subtraction with Significant Figures
When combining measurements with different degrees of accuracy and precision, the accuracy of the final answer can be no greater than the least accurate measurement. This principle can be translated into a simple rule for addition and subtraction: When measurements are added or subtracted, the answer can contain no more decimal places than the least accurate measurement.
| 150.0 g H O | (using significant figures) |
| 150.5 g solution | |
Multiplication and Division With Significant Figures
The same principle governs the use of significant figures in multiplication and division: the final result can be no more accurate than the least accurate measurement. In this case, however, we count the significant figures in each measurement, not the number of decimal places: When measurements are multiplied or divided, the answer can contain no more significant figures than the least accurate measurement.
Example: To illustrate this rule, let's calculate the cost of the copper in an old penny that is pure copper. Let's assume that the penny has a mass of 2.531 grams, that it is essentially pure copper, and that the price of copper is 67 cents per pound. We can start by from grams to pounds.
We then use the price of a pound of copper to calculate the cost of the copper metal.
There are four significant figures in both the mass of the penny (2.531) and the number of grams in a pound (453.6). But there are only two significant figures in the price of copper, so the final answer can only have two significant figures.
| Calculate the length in inches of a piece of wood 1.245 feet long. Determine the correct number of significant figures.
|
Rounding Off
When the answer to a calculation contains too many significant figures, it must be rounded off.
There are 10 digits that can occur in the last decimal place in a calculation. One way of rounding off involves underestimating the answer for five of these digits (0, 1, 2, 3, and 4) and overestimating the answer for the other five (5, 6, 7, 8, and 9). This approach to rounding off is summarized as follows.
If the digit is smaller than 5, drop this digit and leave the remaining number unchanged. Thus, 1.684 becomes 1.68.
If the digit is 5 or larger, drop this digit and add 1 to the preceding digit. Thus, 1.247 becomes 1.25.
Units | Errors | Significant Figures | Scientific Notation
Back to General Chemistry Topic Review
Significant Figures and False Precision
- Published: 01 August 2018
- Volume 39 , pages 367–368, ( 2018 )
Cite this article

- John Morral 1
7133 Accesses
2 Citations
1 Altmetric
Explore all metrics
Avoid common mistakes on your manuscript.

John Morral
A story exists of a museum guide who explains to visitors that several bones on display are 160 million and 5 years old. When questioned about the five extra years, the guide replies, “5 years ago an archeologist told me that the bones were 160 million years old.” Some might view this as an amusing story, but for scientists and engineers it is a cautionary tale about significant figures and false precision.
Significant figures are an important scientific concept in which it is assumed that all significant figures in a number are accurate except for the final digit. The final digit is uncertain either because the number was rounded off or because numbers in experiments or calculations were found to vary.
The numbers 430, 43, 4.3, 0.43 and 0.043 all have two significant figures. The location of decimal points is not a factor. The uncertainty of 43 depends on how it was determined. If it was rounded off, the number is between 42.5 and 43.5 and is written as 43 ± 0.5 with 0.5 being the uncertainty. The uncertainty of other numbers depends on their source. In scientific work, numbers are often the result of averages obtained from multiple experiments. Then the uncertainty is given by the standard deviation rounded off to one digit to obtain the number of significant figures.
When the museum guide gave the age of the bones as 160,000,005 years old, the age became a number with nine significant figures. That is obviously wrong and is an example of false precision. Wiki writes, “False precision (also called overprecision, fake precision, misplaced precision and spurious precision) occurs when numerical data are presented in a manner that implies better precision than is justified.”
I am concerned when seeing manuscripts written with standard deviations having two or more significant figures. As shown in the following example, uncertainties with two or more significant figures add additional digits to the average. The additional digits are uncertain and a result of false precision.
Average | SD | |
|---|---|---|
Experimental numbers | 7.31732 | 0.382521 |
Significant figures with 1-digit uncertainty | 7.3 | 0.4 |
False significant figures with 2-digit uncertainty | 7.32 | 0.38 |
False significant figures with 3-digit uncertainty | 7.317 | 0.383 |
The first line in the example gives the average of raw data obtained from repeated measurements, along with the standard deviation of the average. The second line illustrates how rounding off the standard deviation to one digit determines the number of significant figures in the average value. The last two lines appear to be more accurate, because of their additional digits, but they are a problem. In addition to giving readers a false sense of accuracy, additional digits slow, and possibly prevent, proper interpretation of text and tables by making them unnecessarily complex.
This editorial contains little new information. It is intended to be a reminder that significant figures are extremely important. Whether writing or reviewing a paper, the accuracy and uncertainty of numbers should be a foremost consideration.
Editor-in-Chief
Journal of Phase Equilibria and Diffusion
Author information
Authors and affiliations.
Columbus, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to John Morral .
Rights and permissions
Reprints and permissions
About this article
Morral, J. Significant Figures and False Precision. J. Phase Equilib. Diffus. 39 , 367–368 (2018). https://doi.org/10.1007/s11669-018-0662-z
Download citation
Published : 01 August 2018
Issue Date : August 2018
DOI : https://doi.org/10.1007/s11669-018-0662-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Math Article
Significant Figures

Significant figures are used to establish the number which is presented in the form of digits. These digits carry a meaningful representation of numbers. The term significant digits are also used often instead of figures. We can identify the number of significant digits by counting all the values starting from the 1st non-zero digit located on the left. For example, 12.45 has four significant digits.
The significant figures of a given number are those significant or important digits, which convey the meaning according to its accuracy. For example, 6.658 has four significant digits. These substantial figures provide precision to the numbers. They are also termed as significant digits.
Rules for Significant Figures
- All non-zero digits are significant. 198745 contains six significant digits.
- All zeros that occur between any two non zero digits are significant. For example, 108.0097 contains seven significant digits.
- All zeros that are on the right of a decimal point and also to the left of a non-zero digit is never significant. For example, 0.00798 contained three significant digits.
- All zeros that are on the right of a decimal point are significant, only if, a non-zero digit does not follow them. For example, 20.00 contains four significant digits.
- All the zeros that are on the right of the last non-zero digit, after the decimal point, are significant. For example, 0.0079800 contains five significant digits.
- All the zeros that are on the right of the last non-zero digit are significant if they come from a measurement. For example, 1090 m contains four significant digits.
Rounding Significant Figures
A number is rounded off to the required number of significant digits by leaving one or more digits from the right. When the first digit in left is less than 5, the last digit held should remain constant. When the first digit is greater than 5, the last digit is rounded up. When the digit left is exactly 5, the number held is rounded up or down to receive an even number. When more than one digit is left, rounding off should be done as a whole instead of one digit at a time.
There are two rules to round off the significant numbers:
- First, we have to check, up to which digit the rounding off should be performed. If the number after the rounding off digit is less than 5, then we have to exclude all the numbers present on the right side.
- But if the digit next to the rounding off digit is greater than 5, then we have to add 1 to the rounding off digit and exclude the other numbers on the right side.
Significant Figures Examples
Q.1: Identify the number of significant digits/figures in the following given numbers.
45, 0.046, 7.4220, 5002, 3800
| 45 | Two |
| 0.046 | Two |
| 7.4220 | Five |
| 5002 | Four |
| 3800 | Two |
Q.2: Write 12.378162 correct to 4 significant digits.
The number 12.378162, rounded to 4 significant digits is 12.38
Hence, 12.38 is the answer.
Practice Questions
- Determine the number of significant digits from the following given numbers.
84, 0.084, 5.8480, 2005, 8400
- Solve the following 4.76 + 5.62 + 33.21 and find the number of significant digits/figures.
- Estimate the number of significant digits from the following computations. 5.2 x 10 3 x 6.732 x 10 3
- Write 45.378212 correct to 3 significant digits/figures.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Maths related queries and study materials
Your result is as below
Request OTP on Voice Call
| MATHS Related Links | |
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- Article Information
Data Sharing Statement
- As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss JAMA Medical News & Perspectives May 16, 2023 This Medical News article discusses chronic weight management with semaglutide, sold under the brand names Ozempic and Wegovy. Melissa Suran, PhD, MSJ
- Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists JAMA Special Communication August 15, 2023 This Special Communication used data from the US Food and Drug Administration to analyze how manufacturers of brand-name glucagon-like peptide 1 (GLP-1) receptor agonists have used patent and regulatory systems to extend periods of market exclusivity. Rasha Alhiary, PharmD; Aaron S. Kesselheim, MD, JD, MPH; Sarah Gabriele, LLM, MBE; Reed F. Beall, PhD; S. Sean Tu, JD, PhD; William B. Feldman, MD, DPhil, MPH
- What to Know About Wegovy’s Rare but Serious Adverse Effects JAMA Medical News & Perspectives December 12, 2023 This Medical News article discusses Wegovy, Ozempic, and other GLP-1 receptor agonists used for weight management and type 2 diabetes. Kate Ruder, MSJ
- GLP-1 Receptor Agonists and Gastrointestinal Adverse Events—Reply JAMA Comment & Response March 12, 2024 Ramin Rezaeianzadeh, BSc; Mohit Sodhi, MSc; Mahyar Etminan, PharmD, MSc
- GLP-1 Receptor Agonists and Gastrointestinal Adverse Events JAMA Comment & Response March 12, 2024 Karine Suissa, PhD; Sara J. Cromer, MD; Elisabetta Patorno, MD, DrPH
- GLP-1 Receptor Agonist Use and Risk of Postoperative Complications JAMA Research Letter May 21, 2024 This cohort study evaluates the risk of postoperative respiratory complications among patients with diabetes undergoing surgery who had vs those who had not a prescription fill for glucagon-like peptide 1 receptor agonists. Anjali A. Dixit, MD, MPH; Brian T. Bateman, MD, MS; Mary T. Hawn, MD, MPH; Michelle C. Odden, PhD; Eric C. Sun, MD, PhD
- Glucagon-Like Peptide-1 Receptor Agonist Use and Risk of Gallbladder and Biliary Diseases JAMA Internal Medicine Original Investigation May 1, 2022 This systematic review and meta-analysis of 76 randomized clinical trials examines the effects of glucagon-like peptide-1 receptor agonist use on the risk of gallbladder and biliary diseases. Liyun He, MM; Jialu Wang, MM; Fan Ping, MD; Na Yang, MM; Jingyue Huang, MM; Yuxiu Li, MD; Lingling Xu, MD; Wei Li, MD; Huabing Zhang, MD
- Cholecystitis Associated With the Use of Glucagon-Like Peptide-1 Receptor Agonists JAMA Internal Medicine Research Letter October 1, 2022 This case series identifies cases reported in the US Food and Drug Administration Adverse Event Reporting System of acute cholecystitis associated with use of glucagon-like peptide-1 receptor agonists that did not have gallbladder disease warnings in their labeling. Daniel Woronow, MD; Christine Chamberlain, PharmD; Ali Niak, MD; Mark Avigan, MDCM; Monika Houstoun, PharmD, MPH; Cindy Kortepeter, PharmD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Sodhi M , Rezaeianzadeh R , Kezouh A , Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. 2023;330(18):1795–1797. doi:10.1001/jama.2023.19574
Manage citations:
© 2024
- Permissions
Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss
- 1 Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada
- 2 StatExpert Ltd, Laval, Quebec, Canada
- 3 Department of Ophthalmology and Visual Sciences and Medicine, University of British Columbia, Vancouver, Canada
- Medical News & Perspectives As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss Melissa Suran, PhD, MSJ JAMA
- Special Communication Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists Rasha Alhiary, PharmD; Aaron S. Kesselheim, MD, JD, MPH; Sarah Gabriele, LLM, MBE; Reed F. Beall, PhD; S. Sean Tu, JD, PhD; William B. Feldman, MD, DPhil, MPH JAMA
- Medical News & Perspectives What to Know About Wegovy’s Rare but Serious Adverse Effects Kate Ruder, MSJ JAMA
- Comment & Response GLP-1 Receptor Agonists and Gastrointestinal Adverse Events—Reply Ramin Rezaeianzadeh, BSc; Mohit Sodhi, MSc; Mahyar Etminan, PharmD, MSc JAMA
- Comment & Response GLP-1 Receptor Agonists and Gastrointestinal Adverse Events Karine Suissa, PhD; Sara J. Cromer, MD; Elisabetta Patorno, MD, DrPH JAMA
- Research Letter GLP-1 Receptor Agonist Use and Risk of Postoperative Complications Anjali A. Dixit, MD, MPH; Brian T. Bateman, MD, MS; Mary T. Hawn, MD, MPH; Michelle C. Odden, PhD; Eric C. Sun, MD, PhD JAMA
- Original Investigation Glucagon-Like Peptide-1 Receptor Agonist Use and Risk of Gallbladder and Biliary Diseases Liyun He, MM; Jialu Wang, MM; Fan Ping, MD; Na Yang, MM; Jingyue Huang, MM; Yuxiu Li, MD; Lingling Xu, MD; Wei Li, MD; Huabing Zhang, MD JAMA Internal Medicine
- Research Letter Cholecystitis Associated With the Use of Glucagon-Like Peptide-1 Receptor Agonists Daniel Woronow, MD; Christine Chamberlain, PharmD; Ali Niak, MD; Mark Avigan, MDCM; Monika Houstoun, PharmD, MPH; Cindy Kortepeter, PharmD JAMA Internal Medicine
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss. 1 Studies have found increased risks of gastrointestinal adverse events (biliary disease, 2 pancreatitis, 3 bowel obstruction, 4 and gastroparesis 5 ) in patients with diabetes. 2 - 5 Because such patients have higher baseline risk for gastrointestinal adverse events, risk in patients taking these drugs for other indications may differ. Randomized trials examining efficacy of GLP-1 agonists for weight loss were not designed to capture these events 2 due to small sample sizes and short follow-up. We examined gastrointestinal adverse events associated with GLP-1 agonists used for weight loss in a clinical setting.
We used a random sample of 16 million patients (2006-2020) from the PharMetrics Plus for Academics database (IQVIA), a large health claims database that captures 93% of all outpatient prescriptions and physician diagnoses in the US through the International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10. In our cohort study, we included new users of semaglutide or liraglutide, 2 main GLP-1 agonists, and the active comparator bupropion-naltrexone, a weight loss agent unrelated to GLP-1 agonists. Because semaglutide was marketed for weight loss after the study period (2021), we ensured all GLP-1 agonist and bupropion-naltrexone users had an obesity code in the 90 days prior or up to 30 days after cohort entry, excluding those with a diabetes or antidiabetic drug code.
Patients were observed from first prescription of a study drug to first mutually exclusive incidence (defined as first ICD-9 or ICD-10 code) of biliary disease (including cholecystitis, cholelithiasis, and choledocholithiasis), pancreatitis (including gallstone pancreatitis), bowel obstruction, or gastroparesis (defined as use of a code or a promotility agent). They were followed up to the end of the study period (June 2020) or censored during a switch. Hazard ratios (HRs) from a Cox model were adjusted for age, sex, alcohol use, smoking, hyperlipidemia, abdominal surgery in the previous 30 days, and geographic location, which were identified as common cause variables or risk factors. 6 Two sensitivity analyses were undertaken, one excluding hyperlipidemia (because more semaglutide users had hyperlipidemia) and another including patients without diabetes regardless of having an obesity code. Due to absence of data on body mass index (BMI), the E-value was used to examine how strong unmeasured confounding would need to be to negate observed results, with E-value HRs of at least 2 indicating BMI is unlikely to change study results. Statistical significance was defined as 2-sided 95% CI that did not cross 1. Analyses were performed using SAS version 9.4. Ethics approval was obtained by the University of British Columbia’s clinical research ethics board with a waiver of informed consent.
Our cohort included 4144 liraglutide, 613 semaglutide, and 654 bupropion-naltrexone users. Incidence rates for the 4 outcomes were elevated among GLP-1 agonists compared with bupropion-naltrexone users ( Table 1 ). For example, incidence of biliary disease (per 1000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for bupropion-naltrexone and 4.6, 7.9, and 1.0, respectively, for pancreatitis.
Use of GLP-1 agonists compared with bupropion-naltrexone was associated with increased risk of pancreatitis (adjusted HR, 9.09 [95% CI, 1.25-66.00]), bowel obstruction (HR, 4.22 [95% CI, 1.02-17.40]), and gastroparesis (HR, 3.67 [95% CI, 1.15-11.90) but not biliary disease (HR, 1.50 [95% CI, 0.89-2.53]). Exclusion of hyperlipidemia from the analysis did not change the results ( Table 2 ). Inclusion of GLP-1 agonists regardless of history of obesity reduced HRs and narrowed CIs but did not change the significance of the results ( Table 2 ). E-value HRs did not suggest potential confounding by BMI.
This study found that use of GLP-1 agonists for weight loss compared with use of bupropion-naltrexone was associated with increased risk of pancreatitis, gastroparesis, and bowel obstruction but not biliary disease.
Given the wide use of these drugs, these adverse events, although rare, must be considered by patients who are contemplating using the drugs for weight loss because the risk-benefit calculus for this group might differ from that of those who use them for diabetes. Limitations include that although all GLP-1 agonist users had a record for obesity without diabetes, whether GLP-1 agonists were all used for weight loss is uncertain.
Accepted for Publication: September 11, 2023.
Published Online: October 5, 2023. doi:10.1001/jama.2023.19574
Correction: This article was corrected on December 21, 2023, to update the full name of the database used.
Corresponding Author: Mahyar Etminan, PharmD, MSc, Faculty of Medicine, Departments of Ophthalmology and Visual Sciences and Medicine, The Eye Care Center, University of British Columbia, 2550 Willow St, Room 323, Vancouver, BC V5Z 3N9, Canada ( [email protected] ).
Author Contributions: Dr Etminan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Sodhi, Rezaeianzadeh, Etminan.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Sodhi, Rezaeianzadeh, Etminan.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Kezouh.
Obtained funding: Etminan.
Administrative, technical, or material support: Sodhi.
Supervision: Etminan.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was funded by internal research funds from the Department of Ophthalmology and Visual Sciences, University of British Columbia.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Massive biomolecular shifts occur in our 40s and 60s, Stanford Medicine researchers find
Time marches on predictably, but biological aging is anything but constant, according to a new Stanford Medicine study.
August 14, 2024 - By Rachel Tompa

We undergo two periods of rapid change, averaging around age 44 and age 60, according to a Stanford Medicine study. Ratana21 /Shutterstock.com
If it’s ever felt like everything in your body is breaking down at once, that might not be your imagination. A new Stanford Medicine study shows that many of our molecules and microorganisms dramatically rise or fall in number during our 40s and 60s.
Researchers assessed many thousands of different molecules in people from age 25 to 75, as well as their microbiomes — the bacteria, viruses and fungi that live inside us and on our skin — and found that the abundance of most molecules and microbes do not shift in a gradual, chronological fashion. Rather, we undergo two periods of rapid change during our life span, averaging around age 44 and age 60. A paper describing these findings was published in the journal Nature Aging Aug. 14.
“We’re not just changing gradually over time; there are some really dramatic changes,” said Michael Snyder , PhD, professor of genetics and the study’s senior author. “It turns out the mid-40s is a time of dramatic change, as is the early 60s. And that’s true no matter what class of molecules you look at.”
Xiaotao Shen, PhD, a former Stanford Medicine postdoctoral scholar, was the first author of the study. Shen is now an assistant professor at Nanyang Technological University Singapore.
These big changes likely impact our health — the number of molecules related to cardiovascular disease showed significant changes at both time points, and those related to immune function changed in people in their early 60s.
Abrupt changes in number
Snyder, the Stanford W. Ascherman, MD, FACS Professor in Genetics, and his colleagues were inspired to look at the rate of molecular and microbial shifts by the observation that the risk of developing many age-linked diseases does not rise incrementally along with years. For example, risks for Alzheimer’s disease and cardiovascular disease rise sharply in older age, compared with a gradual increase in risk for those under 60.
The researchers used data from 108 people they’ve been following to better understand the biology of aging. Past insights from this same group of study volunteers include the discovery of four distinct “ ageotypes ,” showing that people’s kidneys, livers, metabolism and immune system age at different rates in different people.

Michael Snyder
The new study analyzed participants who donated blood and other biological samples every few months over the span of several years; the scientists tracked many different kinds of molecules in these samples, including RNA, proteins and metabolites, as well as shifts in the participants’ microbiomes. The researchers tracked age-related changes in more than 135,000 different molecules and microbes, for a total of nearly 250 billion distinct data points.
They found that thousands of molecules and microbes undergo shifts in their abundance, either increasing or decreasing — around 81% of all the molecules they studied showed non-linear fluctuations in number, meaning that they changed more at certain ages than other times. When they looked for clusters of molecules with the largest changes in amount, they found these transformations occurred the most in two time periods: when people were in their mid-40s, and when they were in their early 60s.
Although much research has focused on how different molecules increase or decrease as we age and how biological age may differ from chronological age, very few have looked at the rate of biological aging. That so many dramatic changes happen in the early 60s is perhaps not surprising, Snyder said, as many age-related disease risks and other age-related phenomena are known to increase at that point in life.
The large cluster of changes in the mid-40s was somewhat surprising to the scientists. At first, they assumed that menopause or perimenopause was driving large changes in the women in their study, skewing the whole group. But when they broke out the study group by sex, they found the shift was happening in men in their mid-40s, too.
“This suggests that while menopause or perimenopause may contribute to the changes observed in women in their mid-40s, there are likely other, more significant factors influencing these changes in both men and women. Identifying and studying these factors should be a priority for future research,” Shen said.
Changes may influence health and disease risk
In people in their 40s, significant changes were seen in the number of molecules related to alcohol, caffeine and lipid metabolism; cardiovascular disease; and skin and muscle. In those in their 60s, changes were related to carbohydrate and caffeine metabolism, immune regulation, kidney function, cardiovascular disease, and skin and muscle.
It’s possible some of these changes could be tied to lifestyle or behavioral factors that cluster at these age groups, rather than being driven by biological factors, Snyder said. For example, dysfunction in alcohol metabolism could result from an uptick in alcohol consumption in people’s mid-40s, often a stressful period of life.
The team plans to explore the drivers of these clusters of change. But whatever their causes, the existence of these clusters points to the need for people to pay attention to their health, especially in their 40s and 60s, the researchers said. That could look like increasing exercise to protect your heart and maintain muscle mass at both ages or decreasing alcohol consumption in your 40s as your ability to metabolize alcohol slows.
“I’m a big believer that we should try to adjust our lifestyles while we’re still healthy,” Snyder said.
The study was funded by the National Institutes of Health (grants U54DK102556, R01 DK110186-03, R01HG008164, NIH S10OD020141, UL1 TR001085 and P30DK116074) and the Stanford Data Science Initiative.
- Rachel Tompa Rachel Tompa is a freelance science writer.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Topology optimization with explicit components considering stress constraints.

1. Introduction
2. mmc topology optimization framework and geometrical description, 2.1. mmc topology optimization method, 2.2. a new topology description function, 3. problem formulation, 3.1. problem statement and mathematical formulation, 3.2. global stress control, 4. sensitivity analysis, 5. numerical solution aspects, 5.1. l-shaped beam, 5.2. t-shaped beam, 6. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest, abbreviations.
| SIMP | Solid isotropic material with penalization |
| LSM | Level-set method |
| ESO | Evolutionary structural optimization |
| MMC | Moving morphable components |
| MMA | Method of moving asymptotes |
| TDF | Topology description function |
- Bendsøe, M.P.; Kikuchi, N. Generating optimal topologies in structural design using a homogenization method. Comput. Methods Appl. Mech. Eng. 1988 , 71 , 197–224. [ Google Scholar ] [ CrossRef ]
- Bendsøe, M.P.; Sigmund, O. Material interpolation schemes in topology optimization. Arch. Appl. Mech. 1999 , 69 , 635–654. [ Google Scholar ] [ CrossRef ]
- Querin, O.M.; Young, V.; Steven, G.; Xie, Y. Computational efficiency and validation of bi-directional evolutionary structural optimisation. Comput. Methods Appl. Mech. Eng. 2000 , 189 , 559–573. [ Google Scholar ] [ CrossRef ]
- Wang, M.Y.; Wang, X.; Guo, D. A level set method for structural topology optimization. Comput. Methods Appl. Mech. Eng. 2003 , 192 , 227–246. [ Google Scholar ] [ CrossRef ]
- Guo, X.; Zhang, W.; Zhong, W. Doing topology optimization explicitly and geometrically—A new moving morphable components based framework. J. Appl. Mech. 2014 , 81 , 081009. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Yang, W.; Zhou, J.; Li, D.; Guo, X. Structural topology optimization through explicit boundary evolution. J. Appl. Mech. 2017 , 84 , 011011. [ Google Scholar ] [ CrossRef ]
- Li, Z.; Xu, H.; Zhang, S. A Comprehensive Review of Explicit Topology Optimization Based on Moving Morphable Components (MMC) Method. Arch. Comput. Methods Eng. 2024 , 31 , 1–30. [ Google Scholar ] [ CrossRef ]
- Ruichao, L.; Shikai, J.; Ying, L.; Dengbao, X.; Yang, C. A Hybrid Topology Optimization Method Of Simp And Mmc Considering Precise Control Of Minimum Size. Chin. J. Theor. Appl. Mech. 2022 , 54 , 3524–3537. [ Google Scholar ]
- Zhang, J.; Liao, J.; Liu, E. Topology optimization method based on SIMP-MMC for structure size precise control. J. Mech. Strength 2022 , 44 , 102–110. [ Google Scholar ]
- Cheng, G.; Jiang, Z. Study on topology optimization with stress constraints. Eng. Optim. 1992 , 20 , 129–148. [ Google Scholar ] [ CrossRef ]
- Cheng, G.D.; Guo, X. ε -relaxed approach in structural topology optimization. Struct. Optim. 1997 , 13 , 258–266. [ Google Scholar ] [ CrossRef ]
- Rozvany, G.; Sobieszczanski-Sobieski, J. New optimality criteria methods: Forcing uniqueness of the adjoint strains by corner-rounding at constraint intersections. Struct. Optim. 1992 , 4 , 244–246. [ Google Scholar ] [ CrossRef ]
- Kreisselmeier, G.; Steinhauser, R. Systematic control design by optimizing a vector performance index. In Computer Aided Design of Control Systems ; Elsevier: Amsterdam, The Netherlands, 1980; pp. 113–117. [ Google Scholar ]
- Duysinx, P.; Sigmund, O. New developments in handling stress constraints in optimal material distribution. In Proceedings of the 7th AIAA/USAF/NASA/ISSMO Symposium on Multidisciplinary Analysis and Optimization, St. Louis, MO, USA, 2–4 September 1998; p. 4906. [ Google Scholar ]
- Yang, D.; Liu, H.; Zhang, W.; Li, S. Stress-constrained topology optimization based on maximum stress measures. Comput. Struct. 2018 , 198 , 23–39. [ Google Scholar ] [ CrossRef ]
- Senhora, F.V.; Giraldo-Londono, O.; Menezes, I.F.; Paulino, G.H. Topology optimization with local stress constraints: A stress aggregation-free approach. Struct. Multidiscip. Optim. 2020 , 62 , 1639–1668. [ Google Scholar ] [ CrossRef ]
- Zhai, X.; Chen, F.; Wu, J. Alternating optimization of design and stress for stress-constrained topology optimization. Struct. Multidiscip. Optim. 2021 , 64 , 2323–2342. [ Google Scholar ] [ CrossRef ]
- da Silva, G.A.; Aage, N.; Beck, A.T.; Sigmund, O. Local versus global stress constraint strategies in topology optimization: A comparative study. Int. J. Numer. Methods Eng. 2021 , 122 , 6003–6036. [ Google Scholar ] [ CrossRef ]
- Zhang, S.; Gain, A.L.; Norato, J.A. Stress-based topology optimization with discrete geometric components. Comput. Methods Appl. Mech. Eng. 2017 , 325 , 1–21. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Li, D.; Zhou, J.; Du, Z.; Li, B.; Guo, X. A moving morphable void (MMV)-based explicit approach for topology optimization considering stress constraints. Comput. Methods Appl. Mech. Eng. 2018 , 334 , 381–413. [ Google Scholar ] [ CrossRef ]
- Rostami, P.; Marzbanrad, J. Stress-limited topology optimization with local volume constraint using moving morphable components. Arch. Appl. Mech. 2021 , 91 , 2345–2367. [ Google Scholar ] [ CrossRef ]
- Deng, J.; Chen, W. Design for structural flexibility using connected morphable components based topology optimization. Sci. China Technol. Sci. 2016 , 59 , 839–851. [ Google Scholar ] [ CrossRef ]
- Guo, X.; Zhang, W.; Zhang, J.; Yuan, J. Explicit structural topology optimization based on moving morphable components (MMC) with curved skeletons. Comput. Methods Appl. Mech. Eng. 2016 , 310 , 711–748. [ Google Scholar ] [ CrossRef ]
- Zheng, R.; Kim, C. An enhanced topology optimization approach based on the combined MMC and NURBS-curve boundaries. Int. J. Precis. Eng. Manuf. 2020 , 21 , 1529–1538. [ Google Scholar ] [ CrossRef ]
- Wang, L.; Shi, D.; Zhang, B.; Li, G.; Liu, P. Real-time topology optimization based on deep learning for moving morphable components. Autom. Constr. 2022 , 142 , 104492. [ Google Scholar ] [ CrossRef ]
- Li, Z.; Hu, X.; Chen, W. Moving morphable curved components framework of topology optimization based on the concept of time series. Struct. Multidiscip. Optim. 2023 , 66 , 19. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Yuan, J.; Zhang, J.; Guo, X. A new topology optimization approach based on Moving Morphable Components (MMC) and the ersatz material model. Struct. Multidiscip. Optim. 2016 , 53 , 1243–1260. [ Google Scholar ] [ CrossRef ]
- Ni, B.; Elishakoff, I.; Jiang, C.; Fu, C.; Han, X. Generalization of the super ellipsoid concept and its application in mechanics. Appl. Math. Model. 2016 , 40 , 9427–9444. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Li, D.; Yuan, J.; Song, J.; Guo, X. A new three-dimensional topology optimization method based on moving morphable components (MMCs). Comput. Mech. 2017 , 59 , 647–665. [ Google Scholar ] [ CrossRef ]
- Guo, H.; Zhao, K.; Wang, M.Y. A new approach for simultaneous shape and topology optimization based on dynamic implicit surface function. Control Cybern. 2005 , 34 , 255–282. [ Google Scholar ]
- Shannon, T.; Robinson, T.; Murphy, A.; Armstrong, C. Generalized Bezier components and successive component refinement using moving morphable components. Struct. Multidiscip. Optim. 2022 , 65 , 193. [ Google Scholar ] [ CrossRef ]
- Verbart, A.; Langelaar, M.; Keulen, F.v. A unified aggregation and relaxation approach for stress-constrained topology optimization. Struct. Multidiscip. Optim. 2017 , 55 , 663–679. [ Google Scholar ] [ CrossRef ]
- Cui, T.; Sun, Z.; Liu, C.; Li, L.; Cui, R.; Guo, X. Topology optimization of plate structures using plate element-based moving morphable component (MMC) approach. Acta Mech. Sin. 2020 , 36 , 412–421. [ Google Scholar ] [ CrossRef ]
- Du, Z.; Cui, T.; Liu, C.; Zhang, W.; Guo, Y.; Guo, X. An efficient and easy-to-extend Matlab code of the Moving Morphable Component (MMC) method for three-dimensional topology optimization. Struct. Multidiscip. Optim. 2022 , 65 , 158. [ Google Scholar ] [ CrossRef ]
- Svanberg, K. The method of moving asymptotes—A new method for structural optimization. Int. J. Numer. Methods Eng. 1987 , 24 , 359–373. [ Google Scholar ] [ CrossRef ]
- Zhang, W.S.; Guo, X.; Wang, M.Y.; Wei, P. Optimal topology design of continuum structures with stress concentration alleviation via level set method. Int. J. Numer. Methods Eng. 2013 , 93 , 942–959. [ Google Scholar ] [ CrossRef ]
- Manual, A.S.U. Abaqus 6.11. 2012, Volume 89. v6. Available online: http://130.149.89.49:2080/v2016/index.html (accessed on 17 July 2024).
- Jiang, X.; Wang, H.; Li, Y.; Mo, K. Machine learning based parameter tuning strategy for MMC based topology optimization. Adv. Eng. Softw. 2020 , 149 , 102841. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| epsimin | raa0 | albefa | asyinit | asyincr | asydecr |
|---|---|---|---|---|---|
| 0.01 | 0.4 | 0.1 | 0.8 | 0.6 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Ma, Y.; Li, Z.; Wei, Y.; Yang, K. Topology Optimization with Explicit Components Considering Stress Constraints. Appl. Sci. 2024 , 14 , 7171. https://doi.org/10.3390/app14167171
Ma Y, Li Z, Wei Y, Yang K. Topology Optimization with Explicit Components Considering Stress Constraints. Applied Sciences . 2024; 14(16):7171. https://doi.org/10.3390/app14167171
Ma, Yubao, Zhiguo Li, Yuxuan Wei, and Kai Yang. 2024. "Topology Optimization with Explicit Components Considering Stress Constraints" Applied Sciences 14, no. 16: 7171. https://doi.org/10.3390/app14167171
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Biochem Rev
- v.29(Suppl 1); 2008 Aug

Significant Figures
Tony badrick.
1 Biochemistry Department, Sullivan Nicolaides Pathology, Taringa, Brisbane, Qld 4068
Peter E Hickman
2 Chemical Pathology, ACT Pathology, Canberra 2606, Australia
- For consistency of reporting the same number of significant figures should be used for results and reference intervals.
- The choice of the reporting interval should be based on analytical imprecision (measurement uncertainty).
Introduction
The results of an analysis can be used for a number of different purposes by a clinician. Comparing a result against a reference interval or a clinical decision point can be used to confirm or reject a diagnosis. But often a result is compared with previous results from a particular patient to monitor treatment, be that result a drug level, a glycated haemoglobin level or a creatinine. In this latter case it is the difference between sequential results that contains the information needed, and this difference is conveyed to a large extent by the number of significant figures, or reporting interval, of an analyte. A further complicating factor for a clinician trying to interpret consecutive results is that they may come from different laboratories, so the clinician may be confronted with a pair of results such as 20 and 20.56!
When reporting a result, we may be faced with the problem “is an apparent change in result real, or simply a reflection of the ‘noise’ due to analytical imprecision and biological variation?” The choice of the reporting interval (incremental value chosen for reporting analyte concentration, e.g. for sodium this is 1 mmol/L, whilst for potassium, it is 0.1 mmol/L) should reflect this background noise.
As the measurement systems used in routine chemistry laboratories produce a continuous signal (spectrophotometric, voltage) what happens when a result is rounded is that a series of results are ‘binned’ together. Thus a result of 20 units actually represents the spread of measured results from 19.5 to 20.4. The ‘rounding’ could hide a significant change in result. For example, the range of results from 18.5 to 20.4 would be rounded to 19 and 20 units, a reported change of 1 unit, but a potential difference of up to 1.9. Often, it would appear that the reporting interval chosen is too small which can give a false impression that a change has occurred.
The reporting interval should be such that any result change is greater than the analytical imprecision. The number of significant figures reported should be dependent on analytical imprecision (standard deviation a , SD a ) and, perhaps, biological variation (intra-individual SD, SD i ), but in practice often is quite arbitrary, and it is apparent that for many assays, analyte concentrations are reported to an excessive number of significant figures. 1
Some Relevant Statistics
It is worth reviewing the relevant statistical basis of differences between two numbers which are the results of an analytical process. We assume that the measurements follow a Gaussian distribution, that is, if we repeatedly measured any sample, those results would follow a Gaussian distribution which is described by its mean and SD. That means that approximately 68% of all those repeated values would lie within the mean ±1 SD, that approximately 96% of those values would lie within the mean ±2 SD and 99.7% of values would lie within the mean ±3 SD. These multipliers of the SD, 1, 2 or 3, are called the standard normal deviates or the z-scores. Any measurement of a parameter has an associated analytical error which is described as the analytical standard deviation (SD a ), so any result will lie within the mean ± 2 SD 96% of the time.
Any biological parameter will also have some biological variation associated with it and this is described by a standard deviation (SD i ), so that a parameter will vary day to day around a mean value with an SD of SD i.
Now we are interested in the question: when do two sequential measurements differ? Each of these measurements is subject to analytical error plus normal biological variation. This total ‘normal’ variation for serial results is equal to the sum of the variation about each measurement, that is total variation = variation in measurement 1 + measurement 2 =
We can simplify this to √2 x z x ((SD a ) 2 + (SD i ) 2 ) 1/2
We are interested if the net result difference is greater than these combined effects or greater than
√2 x z x ((SDa) 2 + (SD i ) 2 ) 1/2 which is called the critical difference or reference change value (RCV).
If we want a 95% confidence that two consecutive results truly differ, then their difference must be greater than
2.77 x ((SD a ) 2 + (SD i ) 2 ) 1/2 , the constant ‘2.77’ being derived from √2 times the z statistic which is 1.96.
Different Approaches to Reporting Intervals
There have been various published approaches to determining an appropriate number of significant figures to report. For example, Hawkins and Johnson suggested that a simple rule of thumb to use for choosing the appropriate number of significant figures was that the SD should be ≤ 0.7 of the unit of reporting. 2
For example, for serum Na, if the SD is ≤ 0.7 mmol/L but > 0.07 mmol/L, report to the nearest 0.1 mmol/L. Data on imprecision should be available at this level of detail to allow mathematical manipulation with rounding only at the final step. In the above example for serum Na a 95% significant change value would be 0.66 x 2.77 = 1.82 mmol/L and rounded up to 2 mmol/L for the user.
One can see the error if the SD is reported as “1 mmol/L” and then multiplied by 2.77 to give a significant change value of 3 mmol/L rather than the correct value of 2.
A second approach is to consider what information is ‘lost’ by the rounding process. As mentioned earlier, the effect of rounding can ‘hide’ an actual difference of 1.9 units. Thus, for a single point the appropriate reporting interval is √2 x z x σ/1.9, where z is the z score at (1-p) confidence interval, and σ is the analytical SD. 1
If we take into account the effect of sequential results, then there have been two different approaches reported in the literature. Badrick et al. calculated reporting intervals based only on the analytical imprecision of the method. 3 Thus reference intervals were determined without the SD i term because we need to be able to report when two results are analytically different. The cause of this difference is significant in the interpretation of those results but biological variation may either increase or decrease in disease.
Another approach is to include biological variation in the calculation. Jones has reported the impact of using this calculation (2.77 x √((SD a ) 2 + (SD i ) 2 ) 1/2 ) 4 and has highlighted the impact of ‘binning’ results. Thus, at low concentrations, using a reference interval which is appropriate for the level of imprecision at high concentrations may lead to a loss of clinically useful information. Note that in the paper of Badrick et al., different concentrations with their respective SD a were used rather than a constant imprecision value across the measurement range.
We have concentrated on the critical difference between two sequential results, but many results are singleton and are interpreted against a reference interval or clinical decision point (either empirical or based on evidence). Under these circumstances, the appropriate reference interval should be related to the imprecision of the measurement alone. Thus, the critical difference becomes 2.77 x SD a , for a 95% confidence interval. 3
This discussion assumes that the laboratory has complete control over the way it reports results and their significant figures. However, often this is not the case as there is an intervening Laboratory Information System which may or may not allow rounding or differential rounding based on a threshold concentration.
In the table we have extracted some data from the papers of Hawkins et al. 1 and Badrick et al. 3 The table gives for a range of commonly measured analytes estimates of reporting interval based on different sources. The justifiable unit magnitude (using p=0.05) is calculated using SD for the 50 th centile rankings of all laboratories enrolled in the General Serum Chemistry program from the Royal College of Pathologists of Australasia Quality Assurance Program Pty. Ltd. (RCPA QAP) 5 This represents current ‘state of the art’. The table also gives the reporting unit intervals that are implicit from a well-known clinical chemistry textbook (Tietz Textbook of Clinical Chemistry 6 ), a learned body (RCPA 7 ), and from the data entry sheet for the General Serum Chemistry program from the RCPA QAP. The final column contains the theoretical reporting interval calculated from method of Badrick et al. 3
Justified reporting unit magnitude (p=0.05) based on performance in RCPA General Serum Chemistry QAP Cycle 68, together with unit size taken from various authorities.
| Analyte | Units | Justifiable Unit magnitude from 50% QAP SD | RCPA Unit Size | Tietz Unit Size | QAP Unit Size | RI |
|---|---|---|---|---|---|---|
| mmol/L | 0.07 | 0.01 | 0.01 | 0.01 | 0.05 | |
| μmol/L | 10.83 | 10 | 1 | 1 | 1 | |
| μg/L | 30.86 | 1 | 1 | 1 | 5 @ 56 20 @ 415 | |
| mmol/L | 0.45 | 0.1 | 0.1 | 0.1 | 0.1 @ 3.4 0.5 @ 15.0 | |
| U/L | 21.84 | 1 | 1 | 1 | 5@ 115 10 @ 436 | |
| mmol/L | 0.48 | 0.1 | 0.1 | 0.1 | 0.2 @ 5.5 0.5 @ 18.5 | |
| nmol/L | 47.61 | 1 | 1 | 1 | 10@81 40@550 |
It is apparent from the table that current laboratory reporting interval practice is inappropriate even when only analytical imprecision is considered. A more appropriate approach taking into account laboratory imprecision is seen from the final column.
Even when only analytical imprecision is considered it has been reported that many laboratories use an inappropriate number of significant figures or reporting interval. 1 There is a great need for portability of results and therefore agreement between laboratory information systems in reference intervals and reporting intervals. One of the purposes of the uncertainty of measurement exercise in laboratories should be to critically review the current number of significant figures reported by laboratories and to amend these based on the imprecision of the assay and the biological variation of the analyte. However, we suspect that many laboratories have not taken the opportunity to revise their reporting intervals. When a laboratory does report a result it must be aware that the number of significant figures reported should be carefully considered and be small in comparison to the imprecision and biological variation. We have summarised the current literature and strongly suggest that laboratories ensure that their reporting intervals are fit for the purpose of adding value and not confusion to the differential diagnosis.
Competing Interests: None declared.

IMAGES
COMMENTS
As an example elucidating the abovementioned issues, graphics, and flow diagram in the 'Results' section of a research paper written by the authors of this review article, and published in the World Journal of Urology in the year 2010 (World J Urol 2010;28:17-22.) are shown in Figures 1, and and2 2.
Additionally, this article includes examples of figures that are commonly used in research and their essential components using virtual data. Keywords: Comparative study, Figures, Guidelines, ... For many research papers, the statistical results are illustrated using graphs to support their theory and to enable visual comparisons with other ...
For instance, a mean of n n values has n−−√ n times the precision of the individual values: roughly, include one extra significant figure for 3 ≤ n ≤ 30 3 ≤ n ≤ 30, two for 30 < n ≤ 300 30 < n ≤ 300, etc. (This is rounding on a log-10 scale, obviously.) -Note that the CV does not provide useful information in this regard.
I n the heart of every research article is its data, the information that gives rise to conclusions. However, without proper presentation, the data can be misinterpreted or, worse, ignored. To elude this trap, authors must use well-constructed graphs, figures, and tables to display data and trends, and to summarize information.
Explicitly state the number of significant figures (the abbreviation s.f. is sometimes used): For example "20 000 to 2 s.f." or "20 000 (2 sf)". State the expected variability (precision) explicitly with a plus-minus sign, as in 20 000 ± 1%. This also allows specifying a range of precision in-between powers of ten.
The rules for determining the number of significant figures are as follows: All nonzero digits are significant. For example, the value 211.8 has four significant figures. All zeros that are found between nonzero digits are significant. Thus, the number 20,007, with three 0s between the 2 and 7, has a total of five significant figures.
The results section of a research paper tells the reader what you found, while the discussion section tells the reader what your findings mean. The results section should present the facts in an academic and unbiased manner, avoiding any attempt at analyzing or interpreting the data. Think of the results section as setting the stage for the ...
Types of Figures in Research Paper. There are several types of figures commonly used in research papers, including: Line graphs: These are used to show trends or changes in data over time. Bar graphs: These are used to compare data across different categories or groups. Pie charts: These are used to show proportions or percentages of data.
Rule 4: Refine and repeat until the story is clear. The goal of good figure design is to have your audience clearly understand the main point of your research. That is why the final rule is to spend time refining the figure using the purpose, composition, and color tools so that the final design is clear. It is normal to make 2-3 versions of a ...
As a rule of thumb the appropriate number of significant figures can be obtained by taking the base 10 logarithm of the sample size and rounding to the nearest integer. The base 10 logarithm for a sample size of 100 is 2, for 1,000 is 3, for 10,000 is 4, for 100,000 is 5 and so forth. Please note that if your raw data are very unreliable and ...
An Easy Introduction to Statistical Significance (With Examples) Published on January 7, 2021 by Pritha Bhandari.Revised on June 22, 2023. If a result is statistically significant, that means it's unlikely to be explained solely by chance or random factors.In other words, a statistically significant result has a very low chance of occurring if there were no true effect in a research study.
Transcript. This video teaches significant figures rules, crucial for measurements and calculations. It covers identifying significant digits, including non-zero digits, zeros in between, leading zeros, and trailing zeros. Additionally, it introduces scientific notation for clearer representation of significant figures. Created by Sal Khan.
AboutAbout this video. Transcript. Significant figures are the number of digits in a value, often a measurement, that contribute to the degree of accuracy of the value. We start counting significant figures at the first non-zero digit. Calculate the number of significant figures for an assortment of numbers. Created by Sal Khan.
The discussion of significant figures in result reporting is given relatively little formal attention in the field of laboratory medicine. While a few well-written discussions can be found in the ...
The rule of four gives three decimal places for risk ratios from 0.040 to 0.399, two from 0.40 to 3.99 and one from 4.0 to 39.9. 11 Applying it to the example of 22.68 above gives 22.7 (95% CI 7.5 to 74). Alternatively one can apply the rule with one less significant digit, giving 23 with CI 8 to 70. 11.
We can achieve this by controlling the number of digits, or significant figures, used to report the measurement. Determining the Number of Significant Figures The number of significant figures in a measurement, such as 2.531, is equal to the number of digits that are known with some degree of confidence (2, 5, and 3) plus the last digit (1 ...
In such cases it is necessary to explicitly state the number of significant figures. For example, the number 5,359,783 stated to four significant figures reads 5,360,000. The four zeros are essential as they indicate powers of ten. Just by looking at this number we cannot tell the number of significant figures it represents.
The numbers 430, 43, 4.3, 0.43 and 0.043 all have two significant figures. The location of decimal points is not a factor. The uncertainty of 43 depends on how it was determined. If it was rounded off, the number is between 42.5 and 43.5 and is written as 43 ± 0.5 with 0.5 being the uncertainty. The uncertainty of other numbers depends on ...
When rounding, 3 digits is acceptable if rounding would change the significance of a value (eg, you may write P =.049 instead of .05). If P <.01, it should be expressed to 3 digits. For P values less than .001, report them as P <.001, instead of the actual exact P value. Expressing P to more than 3 significant digits does not add useful ...
Rules for Significant Figures. All non-zero digits are significant. 198745 contains six significant digits. All zeros that occur between any two non zero digits are significant. For example, 108.0097 contains seven significant digits. All zeros that are on the right of a decimal point and also to the left of a non-zero digit is never significant.
The MSC involves collecting electively choosing of the stories that report changes experienced by participants. These stories focus on who did what, when, why, and the reasons why the event was significant from qualitative and participatory perspectives (Serrat, 2017).Rick Davies introduced MSC in 1995 to address the challenges associated with monitoring and evaluating a complex participatory ...
Both groups were observed to have similar increases in gastrocnemius strength at the 8wks (BFR: p=0.004; control: p<0.001) and 16wks postoperative (BFR: p=0.002; control: p<0.001)(Figure 2). However, there was a greater improvement towards achieving limb symmetry in the BFR group at 8wks postoperative (p=0.048) (Figure 2).
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss. 1 Studies have found increased risks of gastrointestinal adverse events (biliary disease, 2 pancreatitis, 3 bowel obstruction, 4 and gastroparesis 5) in patients with diabetes. 2-5 Because such patients have higher baseline risk for ...
Identifying and studying these factors should be a priority for future research," Shen said. Changes may influence health and disease risk. In people in their 40s, significant changes were seen in the number of molecules related to alcohol, caffeine and lipid metabolism; cardiovascular disease; and skin and muscle.
Recent proposals to change the p value threshold from 0.05 to 0.005 or to retire statistical significance altogether have garnered much criticism and debate. 1 2 As of the writing of our manuscript, the proposal to eliminate statistical significance testing, backed by over 800 signatories, achieved record-breaking status on Altmetrics, with an attention score exceeding 13 000 derived from 19 ...
Patients in the cryocompression group showed a significant decrease in post-operative opioid consumption when compared to standard care (median 56.1 OMME [IQR 66.1] vs. median 112 OMME [IQR 99.4]; p=0.02468) (Figure 1). A significant increase in self-reported function (SF-36 Physical Function subscale) was seen in the cryocompression group at 2 ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
1.Introduction. The pear (Pyrus spp.), an member of the Rosaceae family, holds global renown for its palatable taste, nutritional offerings, and significant economic implications (Wang et al., 2023a).Pear is originally from China and has diverse germplasm resources and a long cultivation history. Currently, at least 22 pear species are recognized, with over 5 000 accessions preserved (Wu et al ...
For example, Hawkins and Johnson suggested that a simple rule of thumb to use for choosing the appropriate number of significant figures was that the SD should be ≤ 0.7 of the unit of reporting. 2. For example, for serum Na, if the SD is ≤ 0.7 mmol/L but > 0.07 mmol/L, report to the nearest 0.1 mmol/L. Data on imprecision should be ...
Although further research into the extent to which black bears play a role in the maintenance of the sylvatic cycle and transmission of T. callipaeda eyeworms is needed, ... Figures. Figure 1. Morphologic features of adult female Thelazia callipaeda eyeworm isolated from an American black bear in Coolbaugh Township, Monroe County, Pennsylvania ...