

Different Types of Dyes and Their Properties
In order to grasp the concept of dyeing, one needs to have a clear understanding of the different types of dyes that are currently available on the market. Before getting into that, let’s explore the most obvious question first–
What is Dye?
A dye is a coloring material that is used for imparting color to different substances or altering the color of something. Dyes have chromophores and auxochromes which are responsible for their color and substantivity.
Types of Dyes
Dyes can be classified based on various parameters. However, we’ll take a look at four most pronounces ones –
Based on the Source
- Natural dyes
- Synthetic dyes
Based on Ionic Nature
- Anionic dyes: Reactive dye, acid dye, etc.
- Cationic dyes: Basic dye.
- Non-ionic dyes: Disperse dye.
Based on Chromophoric Groups
- Anthraquinone dyes
- Nitro and nitroso dyes
- Triarylmethane dyes
- Indigo dyes
Based on Chemical Structure
Ready-made dyes.
- Water-soluble dyes: Reactive dye, direct dye, Acid dye, etc.
- Water-insoluble dyes: Vat dye, Sulphur dye, etc.
Ingrain Dyes
- Mineral colors
- Azoic colors
- Oxidation colors
Basic Theory of Dyeing
In dyeing, color is transferred to a textile material to make it permanently colored. In the textile industry, generally, it is done by different machines and consists of several steps.
Now coming to the basic dyeing theory, the interaction between dyes and the textile material along with different dyeing auxiliaries consists of 3 general stages.
- Dye migration from the solution to the surface of the fiber, which can also be called as adsorption.
- Dye diffusion to the interior of the fiber from the surface, which can also be called as absorption.
- Dye fixation by different types of bonds or entrapping inside the fiber pores.
Different types of methods are available for textile coloration.
- Direct dyeing
- Yarn dyeing
- Garment dyeing
- Stock dyeing
- Piece dyeing
- Dope dyeing
Different Dyes with Their Properties
Now, we will discuss different dyes with their primary dyeing mechanism.
Direct Dyes
We can apply these dyes directly on the fiber as they have a strong affinity and so they are called direct dyes. Mainly these dyes are sodium salts of sulphonic acid or carboxylic acid, and their leading chromophoric group is azo. They are also known as substantive dyes.
Properties of Direct Dyes
- They are water-soluble and anionic.
- The dyeing process requires electrolytes and alkaline conditions.
- Generally, cellulosic fiber is dyed, but protein fiber can also be used.
- Fastness properties are average; mainly, the wet fastness is poor. For improved fastness, after-treatment is required.
- They are attached to the fiber with weak hydrogen bonds and Van der Waals’ force of attraction.
- Generally cheap when compared to other dyes.
Primary Mechanism of Direct Dyes
The dyeing involves three steps, adsorption, diffusion, and migration. In water, the cellulosic fiber’s surface becomes anionic. As the dye is also anionic, for neutralizing the surface to remove the repulsion electrolyte is necessary.
Due to swelling in water, the pores of cellulosic fiber’s amorphous region get opened, and the dyes get diffused inside the pores. Dyes are fixed in the fiber with weak H-bond and Van der Waals force. So the fastness properties are not good enough.
Application: Cotton and Viscose.
Reactive Dyes
Reactive dyes have a halogen-containing reactive group present in their structure and become an integral part of the fiber structure by creating a covalent bond. This is the type that is mostly used for dyeing cotton fabrics.
Properties of Reactive Dyes
- These are water-soluble and anionic
- Exhibit good wash and lightfastness and moderate rubbing fastness
- Get fixed into the fiber by a covalent bond
- They are found in powder, liquid, and paste form
- Dyeing requires alkaline conditions and the use of electrolytes.
Primary Mechanism of Reactive Dyes

The primary dyeing mechanism consists of three stages.
- Exhaustion : With the help of electrolytes.
- Fixation : With the help of alkali.
- Washing-off : With the help of a soaping agent.
They generally form two types of reaction with cellulose.
Nucleophilic Substitution Reaction
Cell-OH + HO – ⇒ Cell-O – + H 2 O
Cell-O – + Dye-Cl ⇒ Cell-O-Dye + Cl –
Nucleophilic Addition Reaction
Cell-O – + Dye-SO 2 -CH=CH 2 ⇒ Dye-SO 2 -CH=CH 2 -O-Cell
Application: Mainly cotton, but protein and polyamide can also be dyed.
The application of acid dyes requires an acidic bath. They are mainly carboxylic or sulphuric acid salts. These are the types that are mainly used for dyeing protein fibers.
Properties of Acid Dyes
- They are highly water-soluble and anionic.
- They have substantivity towards polyamide and protein fibers.
- They mainly create ionic bonds, but van der Waals and H-bond also contribute.
- The active colored component is the dye anion.
- They show poor wash fastness, but lightfastness is excellent.
- Acidic medium is required for their application.
Primary Mechanism of Acid Dyes
Wool can be represented as,
H 2 N—W—COOH
In water, under a particular condition,
H 2 N — W — COOH ⇒ H 3 N (+) —W—COO (–)
When acid is added,
HCl ⇒ H + + Cl –
H 3 N + —W—COO – +H + + Cl – ⇒ Cl –+ H 3 N—W—COOH
After the addition of dyes with wool, the following reaction takes place:
H 3 N +− Cl—W—COOH + R– SO 3 −+ Na ⇒ R– SO 3 −+ H 3 N—W—COOH + NaCl
Wool in Acidic Medium + Acid Dye ⇒ Dyed Wool + Salt
Application: Mainly nylon , silk, and wool.
They are mainly salts of organic bases. The cationic part of the dye structure is responsible for color production.
Properties of Basic Dyes
- They are cationic dyes.
- Soluble in alcohol and methylated spirit but not readily soluble in water.
- They exhibit excellent shades but inferior leveling properties.
- They are mainly applicable to jute and acrylic.
- They show average to good fastness properties.
Primary Mechanism of Basic Dyes
The dyeing of acrylic fiber with these dyes is carried out in a weakly acidic condition where acidic acid and sodium acetate is used with a wetting agent, dispersing agent, and sequestering agent. The temperature and pH should be strictly maintained to prevent unlevel dyeing.
The initial uptake of dyes is very high, so a retarding agent is necessary.
Generally, dyeing is carried out at 98°c, and slow cooling is necessary to prevent the formation of creases. Finally, after-treatment with a non-ionic detergent, acetic acid, and a softening agent is carried out.
Application: Best for dyeing acrylic yarn and fabric ; can be used to dye jute also.
These dyes generally consist of a keto group in their structure and made water-soluble by vatting. Their application process of vat dyes is quite similar to sulfur dyes. They are mainly used for dyeing denim or jeans.
Properties of Vat Dyes
- They are natural coloring substances and not soluble in water.
- Vatting is required to convert them into a water-soluble form, and vatting require alkaline conditions.
- They produce a wide range of colors and brilliant shades.
- They exhibit excellent fastness properties except for the rubbing fastness.
- They are found in powder and paste form.
- They are very expensive, and their application is limited.
Primary Mechanism of Vat Dyes
The dyeing contains four main stages –
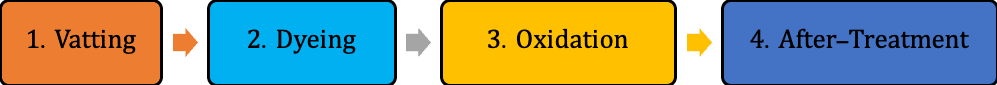
- Vatting: The insoluble dyes are converted into soluble leuco forms by reduction.
- Diffusion: The soluble leuco dyes get inside the cellulosic fiber.
- Oxidation: The soluble dyes converted into insoluble form again.
- Washing-off: The unfixed dyes are removed from the surface.
Application: Cotton.
Sulfur Dyes
Sulfur dyes are quite similar to vat dyes. They are hugely used for producing black and brown shades in cotton. They have a disulfide linkage in their structure.
Properties of Sulfur Dyes
- They are water-insoluble, and the use of reducing agents is required for making them soluble.
- Applied in alkaline condition and oxidation is required for color production.
- Electrolytes can improve the exhaustion.
- They show average fastness properties.
- Range of shades are limited, and generally dull shades are produced.
- They are cheap.
Primary Mechanism of Sulfur Dyes
In order to dye with sulfur dyes, you have to follow the following route –
Application: Cotton and viscose.
Disperse Dyes
Disperse dyes are sparingly water-soluble dyes used for dyeing hydrophobic thermoplastic fibers. These synthetic fabric dyes are mainly substituted azo, anthraquinone, or diphenylamine compounds.
Properties of Disperse Dyes
- They have low molecular weight, are non-ionic, and sparingly water-soluble.
- The dispersing agent is required for making dispersed in water.
- They sublime without decomposition and show gas fume fading.
- They are found in powder and liquid forms.
- They have no strong solubilizing group present in their structurer.
- They do not have an affinity towards any fiber.
- They are physically trapped inside the pores of polyester.
Primary Mechanism of Disperse Dyes
The dyeing of Man-Made Fiber (MMF) with disperse dyes follows these steps –
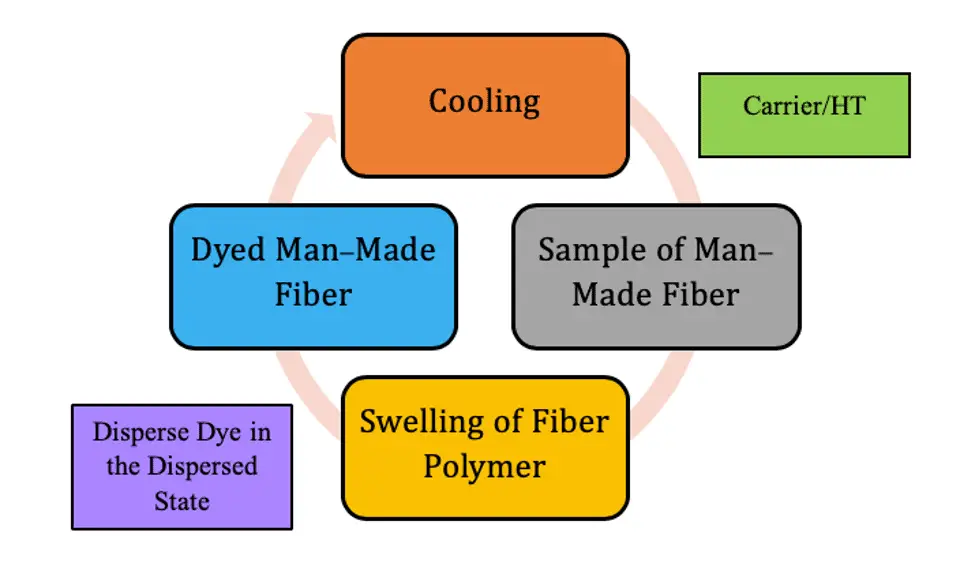
- Dissolution or dispersion of the dye molecules in water
- Diffusion of the dye molecules in the fiber interior.
Application: Acetate, triacetate, nylon, polyester, acrylic.
They are mainly mono or bis azo water-insoluble coloring compounds. They require coupling components for producing colors. The most exciting thing is that they are not ready-made colors like other dyes.
Properties of Azoic Dyes
- They have an insoluble azo group present in their structure and are not water-soluble.
- Two baths, a developing bath, and an impregnation bath are required.
- They produce intense and bright orange, red and scarlet shades.
- They exhibit excellent washing and lightfastness.
- Their actual color depends on diazonium and coupling compounds.
Primary Mechanism of Azoic Dyes
They require two main components.
- Coupling component (Naphthol)
- Diazonium salt
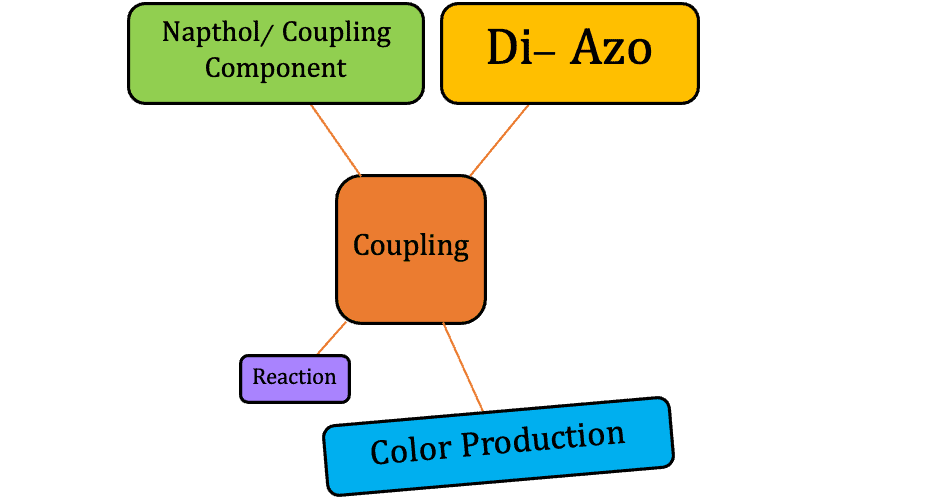
The necessary steps of dyeing are,
- Naphtholation: The insoluble naphthol is made water-soluble by alkali.
- Diazotization: Sodium nitrate reacts with a base containing an amino group that produces diazonium chloride of the base.
- Coupling: The material impregnated in the naphthol solution is added to the diazonium bath to produce color.
Application: Cotton, nylon, polyester.
Mordant Dyes
These dyes have no substantivity towards textile material. Chemical binding agents called mordants help them to get attached to the fiber. They are also called chrome dyes, as they are mainly inorganic chromium.
Properties of Mordant Dyes
- They do not have an affinity for textile material.
- They can produce dark shades.
- They exhibit good fastness properties and leveling properties.
- Dye fiber bond is strong ionic bonds.
- They have a metal ion present in their structure.
- They are soluble in cold water.
Primary Mechanism of Mordant Dyes
A complex is formed with the dyes by the chromium ion when potassium dichromate is added to the solution of acid mordant dyes in the presence of H 2 SO 4 . The complex is water-insoluble and gets precipitated in the fiber.
Three processes can be followed.
- Pre-mordanting also known as Chrome
- Meta mordanting also known as Metachrome
- Post mordanting also known as Afterchrome
Application: Natural protein fibers, nylon, and modacrylic fibers.
Summary of the Property & Uses of Dyes
Before finishing this article let’s summarize the most important attributes of these dyes –
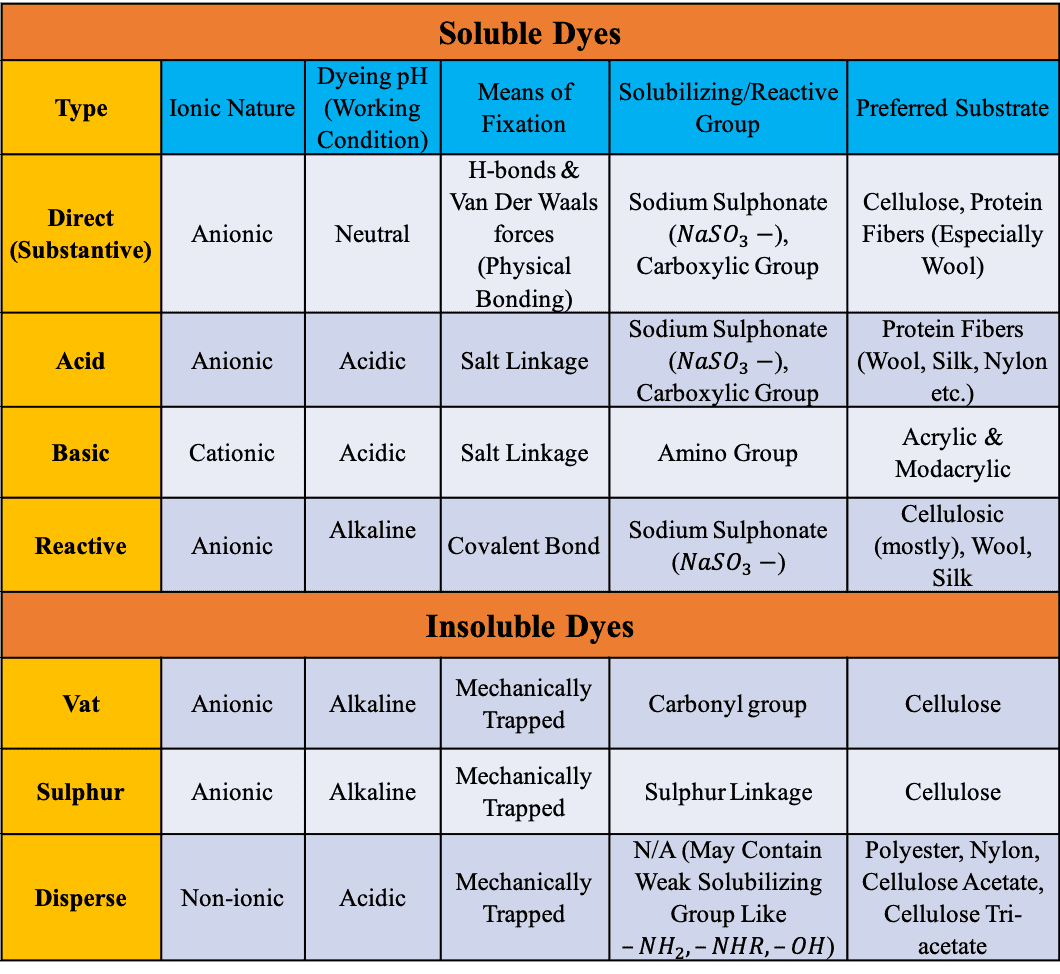
Direct Dye vs Reactive Dye
Here’s the difference between direct dye and reactive dye –
Vat Dye vs Reactive Dye
Here are the most obvious differences between vat dye and reactive dye –
Reactive Dye vs Disperse Dye
The difference between reactive and disperse dyes is that –
Frequently Asked Questions
1. which types of colorants have the best wash fastness.
It varies depending on the material. For example, both are reactive dyes, and vat dyes have excellent wash fasteners on cellulosic fibers as well as cotton and tetron . However, their dyeing procedure is very different.
2. What is the most suitable kind of dyes for dyeing cotton?
That would be fiber reactive dyes. They give a wide variety of shades, possess excellent wash fastness and comes at a cheaper price too.
3. What are the best types of dyes for dyeing polyester?
When it comes to polyester, disperse dyes should be your go-to choice. They require a higher dyeing temperature, though. We have a detailed article on it. Give it a read to know more.
- Introduction to textile engineering by Dr. Hosne Ara Begum
- Houser, Textile dyeing: InTech, Croatia, 2011
- Encyclopedia Britannica: Dyeing and Printing
- Basic principles of textile coloration by A.D. Broadbent
- Textile preparation and dyeing by A.K.R. Choudhury
- Fundamentals and practices in coloration by J.N. Chakraborty
Clothing Technology Expert
I’m Sifat. I work as a lecturer in the Department of Textile Engineering at Port City International University. I’m fascinated by the latest developments in the world of fashion and clothing, so I spend most of my time writing about them on this blog.
I also enjoy singing and playing games on the internet in my free time – though I’m not very good at either!
Search This Blog
Sciencedoze: science, education and technology.
Sciencedoze is a free learning platform where you can explore blogs of Science, Education and Technology. You can follow us on Facebook to be a member of this community.
10 Types of Dyes with their Properties and Dyeing Mechanism
What is a dye.
A dye is a coloring substance that is used for giving color to different substances or altering the color of something. A dye chemically bonds to the substrate to which it is being applied and has an affinity to adhere to the solvent medium.
A dye contains two groups that are chromophore and auxochrome groups in which a chromophore is a color-bearing group that is responsible for dye color whereas auxochrome is a color helper which is responsible for dye fiber reaction.
Examples of dyes: Indigo dye , Phthalein dyes , Alizarin , etc.
10 Types of Dyes
1. reactive dyes.
Reactive dyes are those dyes that form a covalent bond between the dye and fiber. These dyes have a reactive group like halo-heterocycle or an activated double bond which once applied to a fiber with a hydroxyl group on the cellulosic fiber.
In a reactive dye, chromophore has a substituent that is activated and allowed to directly react to the surface of the substrate. Some common examples of reactive dyes are Reactive Blue 5, Procion, Primazin, Levafix, etc.
Dyeing mechanism of Reactive Dyes
The dyeing of material with reactive dye takes place in three stages:
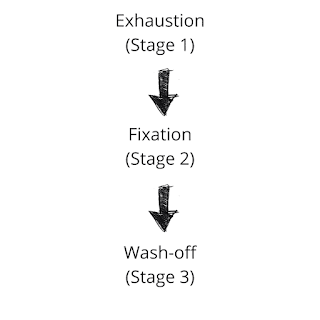
1. Exhaustion
When the fiber is immersed in a dye liquor, an electrolyte is added to assist the exhaustion of Dye. Generally, NaCl is used as an electrolyte that neutralizes the cotton and helps absorption. So, when the textile material is introduced to dye liquor, the dye is exhausted on the fiber.
2. Fixation
Fixation of dye is the reaction of a reactive group of dye with terminal -OH or -NH₂ group of fiber and thus forming a strong covalent bond with it. This is a crucial phase that is controlled by maintaining proper pH by adding alkali. The alkali is used to create proper pH in the dye bath and works as the dye-fixing agent.
There are mainly two types of reaction that occurs at this stage:

3. Wash-off
After dyeing is completed, a good wash should be applied to the material to remove extra and unfixed dyes from the material surface. This is necessary for levelling and good wash-fastness properties. It is done by a series of hot wash, cold wash, and soap solution wash.
Properties of Reactive dyes.
1. Reactive dyes are found in powder, liquid, and print paste form with all types of shades available.
2. Reactive dyes exhibit good wash and lightfastness with a rating of 5 but have moderate rubbing fastness.
3. Reactive dyes are soluble in water and anionic in nature.
4. Reactive dyes have good perspiration fastness with a rating of 4-5.
Application of Reactive dyes
1. Reactive dyes are used for dyeing cotton or other cellulose fibers at home or in the art studio.
2. Reactive dyes are used for silk painting, screen printing, polychromatic printing, and fabric painting.
2. Acid dyes
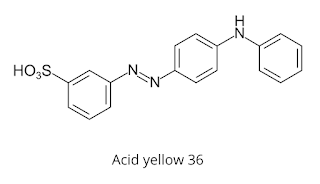
Acid dyes are water-soluble anionic dyes containing sulphonic acid groups (usually present as sodium sulphonate salts). It is applied to fibers like silk, wool, nylon, and modified acrylic fibers using neutral to slightly acidic dye baths. Acid dyes are inexpensive and have a fair light fastness but poor wash fastness.
For example, Acid yellow 36
Dyeing mechanism of Acid dyes
Here we have taken dyeing of wool using an acid dye. So based on the chemical structure of wool, it can be simply represented as H₂N-W-COOH , where 'W' denotes the main non-reacting body of the wool structure. Following are the steps were taken during the dyeing of wool using an acid dye:
1. Submission of wool in water
2. addition of acid, 3. addition of dye, properties of acid dyes.
1. Acid dyes have substantivity towards protein and polyamide fibers.
2. Wash fastness of acid dye is poor but lightfastness is good.
3. Acid dyes are highly water-soluble and anionic.
4. Acid dyes create ionic bonds with fiber but hydrogen bond and van der Waals forces also contribute.
5. Acid dyes have no affinity for cotton cellulose, hence not suitable for cellulosic fibers.
Application of Acid dyes
1. Acid dyes are used for dyeing animal hair fibers like wool, alpaca, and mohair. They are also effective on silk but least effective on cotton fabrics.
2. Acid dyes are also used in histology to color basic tissue proteins. Lee's stain, Eosin stain are some examples of Acid dyes used in histology.
3. Acid dyes are used for food coloring of cookies, bread, drinks, etc to increase the attractiveness of foods that appeal to more customers. Examples of acid dyes used in food are erythrosine , tartrazine , sunset yellow , and allura red.
3. Basic Dyes
Basic Dyes are water water-insoluble cationic dyes that contain amino or alkylamino groups having a positive charge which reacts with negatively charged material. Therefore, the primary way of the coloration process with basic dyes is by ionic bonding. Basic Dyes are bright but not very fast to light, washing, and perspiration. They are mainly applied to acrylic fibers.
For example, Basic Brown 1
Dyeing mechanism of Basic Dyes
If we take the case of dyeing of acrylic fiber, it is the anionic property of acrylic fiber which makes it suitable for dyeing with basic dyes (cationic dyes) . Since there will be a strong ionic interaction between basic dye and fiber.
Action on acrylic of Basic Dyes
Properties of basic dyes.
1. Basic Dyes are not soluble in water but soluble in alcohol and methylated spirit.
2. Basic Dyes have poor leveling properties but average fastness properties.
3. Basic Dyes produce very brilliant shades in which the cationic part of the salt is responsible for color production.
4. Basic Dyes have no affinity for cotton but dyeing of cotton can be carried out by mordanting, fixing, and dyeing operation.
Application of Basic Dyes
1. Basic Dyes are used for jute dyeing and jute printing but wool, acrylic fibers can also be dyed using Basic Dyes.
2. Basic Dyes can even color materials like glass, plastic, porcelain, and sealant of sinks.
3. Basic Dyes used in discharge printing, preparing leather, paper, wood, and straw.
4. Basic Dyes can even dye body skin due to negatively charged nucleic acids of cells.
4. Direct Dyes
As the name suggests, Direct dyes can be applied directly to the fabrics from an aqueous solution. They are also known as substantive dyes and are most frequently used as paper dyes.
Direct dyes have sodium salts of Sulphonic acid or carboxylic acid which makes them water-soluble. They also have an azo linkage (-N=N-) which is a leading chromophoric group. The unique property of Direct dyes is that they don't require mordant or binder during dyeing of cotton.
Foe example, Direct Orange 26
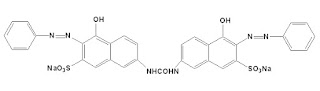
Dyeing mechanism of Direct dyes
Direct dyeing is normally carried out in a neutral or slightly alkaline dye bath at boiling point with the addition of either Sodium Chloride (NaCl), Sodium Sulfate (Na₂SO₄), or Sodium Carbonate (Na₂CO₃). The dyeing takes place in three steps that is adsorption , diffusion , and migration .
So, in water, the cellulosic fiber's surface becomes anionic. As the dye is also anionic, neutralizing the surface to remove the repulsion electrolyte is necessary.
Due to swelling in water, the pores of cellulosic fiber's amorphous region get opened and the dyes get diffused inside the pores. Finally, dyes are fixed in the fiber with weak Hydrogen bonds and van der Waals forces of attraction.
Properties of Direct dyes
1. Direct dyes are water-soluble and anionic.
2. Direct dyes are attached to the fiber with weak hydrogen bonds van der Waals forces of attraction.
3. The colors are not brilliant and the fastness properties of direct dyes are also average. For improved fastness, after-treatment is required.
4. The dyeing process requires electrolytes and alkaline solutions.
Application of Direct dyes
1. Direct dyes are used on cotton, paper, leather, wool, silk, and nylon.
2. Direct dyes are also used as pH indicators and as well as biological stains.
3. Direct dyes are used for dyeing dressing gowns and bedspreads which are not washed properly
4. Direct dyes can be applied well at low temperatures and therefore suitable for tie-dyeing and batik work.
5. Azoic Dyes
The dyes containing at least one insoluble azo group (-N=N-) attached to one or two aromatic rings are called azoic dyes. These dyes are not found in readymade form like other dyes and produced by a reaction between two components that are coupling compound (Naphthol) and Di-azo compound or diazo base or diazo salt.
For example, Bluish red azoic dye

These dyes are used primarily for bright red shades in dyeing and printing because most other classes of fast dyes are lacking in good red dyes.
Dyeing mechanism of Azoic Dye
Following steps are taken during the dyeing of material with Azoic dyes.
1. Naphtholation
In this step, Naphthols which are insoluble in water are converted into the water-soluble compound by treating with alkali.
2. Diazotization
In this step, a base containing an amino group (-NH₂) reacts with the NaNO₂ (Sodium nitrate) to form a solution of diazonium chloride of that base in the presence of excess HCl at 0-5 ℃ temperature.
3. Coupling / Developing
In this final step, the impregnated material is treated in a bath containing diazonium solution to carry out the coupling and thus color is produced inside the fabric.
Properties of Azoic Dyes
1. Azoic dyes are cheap and exhibit excellent washing and lightfastness.
2. Azoic dyes produce intense and bright orange, red and scarlet shades.
3. Azoic dyes have an insoluble azo group present in their structure and hence, they are not water-soluble.
4. The actual color of these dyes depends on diazonium and coupling compounds.
Applications of Azoic dyes
1. Azoic dyes are suitable for cellulose fibers and can be used successfully on protein fibers.
2. Azoic dyes are used widely all over Asia and Australia for batik work.
3. Azoic dyes can be used to give interesting texture color effects on fabric, thread, or paper.
4. Azoic dyes can be used for straight silk painting but it is not advisable due to difficulty in achieving evenness.
6. Disperse Dyes
Disperse dyes are non-ionic , readymade and they are not soluble in water. They are organic coloring substances, suitable for dyeing hydrophobic fibers. Disperse dyes are used for dyeing artificial cellulose ester and synthetic fibers especially acetate and polyester fibers and sometimes nylon and acrylic fibers.
For example, Disperse red 4
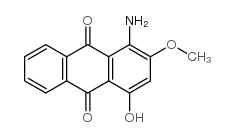
Dyeing mechanism of Disperse dyes
The dyeing of material with Disperse dyes takes place in the following simultaneous steps:
1. Diffusion
In the first step, diffusion of dye in the solid phase into water takes place in which breaks up into individual molecules. This diffusion depends on the dispersibility and solubility of dyestuff which is supported by the presence of dispersing agents and increasing temperature.
2. Adsorption
In the second step, adsorption of the dissolved dye from the solution onto the fiber surface takes place. This dyestuff adsorption by fiber surface is influenced by the solubility of the dye in the dye bath.
3. Diffusion of the adsorbed dye
In the final step, adsorbed dye is diffused from the fiber surface into the interior of the fiber substance towards the center. In normal conditions, the adsorption rate is always higher than the diffusion rate.
Properties of Disperse dyes
1. Disperse dyes have low molecular weight, non-ionic, and sparingly soluble in water.
2. Disperse dyes have fair to good light fastness with a rating of about 4-5 whereas wash fastness of these dyes is moderate to good with a rating of about 3-4 .
3. Disperse dyes tend to sublime without decomposition due to the absence of ionizable groups. That's why the color of disperse-dyed fabric may fade while ironing.
4. Disperse dyes do not undergo any chemical change during dyeing.
Application of Disperse dyes
1. Disperse dyes are widely used for heat transfer printing (Polysol) in which dye is printed onto paper and heat pressed onto the fabric.
2. Disperse dyes are used to dye cellulose diacetate, cellulose triacetate, and polyester fibers.
3. They can also be applied to nylon and acrylic fibers.
7. Mordant Dyes
Mordant dyes are those dye that requires a binding agent known as mordant for their applications. Mordant acts as a binding agent between the fiber and dye which helps the dye to get attached to the fiber. Mordant dyes are also known as chrome dyes as it contains dichromates and chromium complexes.
Most dyes yield different colors with different mordants and can be used with wool, wool blends, silk, cotton, and certain modified-cellulose fibers.
For example, Mordant red 11

Dyeing mechanism of Mordant dyes
When potassium dichromate (K₂Cr₂O₇) is added to the solution of acid-mordant dyes in the presence of H₂SO₄ , a complex is formed with the dyes by chromium ion. The resulting complex is water-insoluble and gets precipitated in the fiber.
Following are the three methods used for mordanting:
1. Pre-mordanting (Chrome)
In this process, the substance is treated with the mordant and then with the dye. The complex between the mordant and dye is formed on the fiber. In this process, the material is impregnated with an insoluble chromium hydrate and then dyeing is done in a separate bath.
2. Meta-mordanting (metachrome)
In this process, dyeing and mordanting are carried out simultaneously in the same bath. The pH of the bath is kept around 6-7 and here the mordant is present in the form of chromate which does not form the lake with the dye and is gradually converted into dichromate.
3. Post-mordanting (After Chrome)
This is one of the oldest and most common mordant dyeing process. Here, the material is first dyed with an acidic dye, and then mordanting with chromium is done in a separate bath or mordanting can also be done in the same bath after exhaustion of the dye has been completed.
Properties of Mordant dyes
1. Mordant dyes exhibit very good light and wash fastness properties with a rating of 4-6 .
2. Mordant dyes can combine with metallic oxides to form color lakes
3. Mordant dyes have no affinity for textile fibers instead they attach to fibers with the help of mordants.
4. Mordant dyes have a metal ion (mainly chromium) as a central atom in the structure which is bonded to neighboring -OH , -COOH , or azo group.
Applications of Mordant dyes
1. Mordant dyes are mostly used on natural protein fibers, nylon, and modacrylic fibers.
2. Mordant dyes are also used for the dyeing of cotton and wool.

8. Vat Dyes
Vat dyes are the insoluble complex of polycyclic molecules that are based on the quinone structure (keto forms) . Vat dyes are synthesised from indigo, anthraquinone and carbazole. The term vat comes from the old indigo method of dyeing in a vat in which reduction of indigo plants takes place through fermentation.
For example, Vat Blue 4
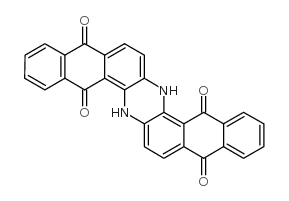
Dyeing mechanism of Vat dyes
The dyeing of Vat dyes takes place in four stages.
In the first stage, the insoluble dyes are converted into soluble leuco forms by reduction.
2. Diffusion
In this stage, the soluble leuco dyes get inside the cellulosic fiber.
3. Oxidation
In this stage, the soluble dyes are converted into insoluble form again.
4. Washing-off
In the final stage, the unfixed dyes are removed from the surface of the fiber.
Properties of Vat dyes
1. Except for rubbing fastness, Vat dyes exhibit excellent washing, light, and perspiration fastness.
2. Vat dyes are very expensive but the leveling properties are excellent.
2. Vat dyes are found in powder and paste form in which powder form is less stable and paste form is stable in dark places.
4. Vat dyes are moderately soluble in hot water but the solubility can be improved by mixing urea in it at 50-60 ℃ temperature.
Applications of Vat dyes
1. Vat dyes are used in the dyeing of cotton, linen, rayon, wool, silk, and sometimes nylon.
2. Vat dyes are used for dyeing uniforms for armed forces, police, fire, etc which are subjected to severe laundry washing and beaching.
3. Vat dyes are used in outdoor fabrics like parasols, tenting, tarpaulins, etc which require high weather fastness.
4. Vat dyes are used in yarns like sewing threads and color threads for weaving.
9. Sulphur Dyes
Sulphur dyes are usually used to dye cellulosic material and hugely used for producing black and brown shades in cotton. The interaction between the fiber and sulphur dye is established through very strong ionic bonds; which are formed between the anionic groups of the dye and ammonium cations of the fiber.
For example, Sulphur red 7
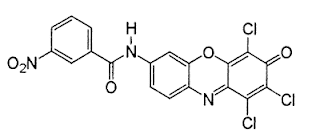
Moreover, sulphur dyes are water-insoluble dyes and get easily solubilized when it is treated with a weak alkaline solution of sodium sulphide or by any other reducing agent to form leuco compound.
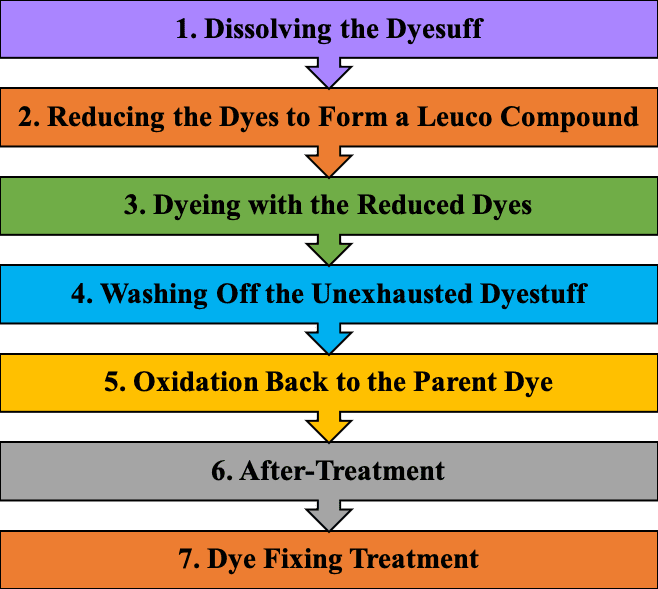
Dyeing mechanism of Sulphur dye
To dye fiber with sulphur dye, the following steps are taken:
1. Dissolving the dyestuff.
2. Reducing the dye to produce a leuco compound.
3. Dyeing with reduced dyes.
4. Washing off the unexhausted dyestuff.
5. Oxidation back to the parent dye.
6. After treatment.
7. Dye fixing treatment.
Properties of Sulphur dyes
1. Sulphur dyes have good leveling and colorfastness properties.
2. Sulphur dyes are cheap and generally produce dark shades like dark greens, dark blues, and blacks.
3. Sulphur dyes are water-insoluble and reducing agents are required to make them soluble.
4. Sulphur dyes are applied in alkali conditions and oxidation is required for color production.
Applications of Sulphur dyes
1. They are used to dye cotton, linen, and rayon fibers.
2. These dyes are used for jigger, winch, and package dyeing of viscose-rayon.
3. They are used to dye khaki color cloths which are used by central armed police forces.
10. Rosaniline Dye
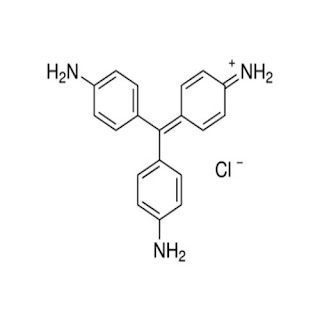
Applications of Rosaniline
Popular posts, crime scene: definition, types and characteristics.

Conducting Polymers: Definition, Examples, Properties and Applications

Documentation of the Crime Scene: Step by Step


What Are The Different Types Of Dyes And How They’re Made?
In the textile industry, the most prevalent types of dyes are reactive, direct, vat, and acid dyes. Reactive dyes form a strong bond with fibers, ensuring durability and resistance to fading. Direct dyes, easily soluble in water, are ideal for cellulose fibers like cotton. Vat dyes, known for their colorfastness, are used in denim production. Acid dyes, on the other hand, are perfect for protein fibers such as wool and silk, offering vibrant coloration.
Key Takeaway
- Acid Dyes: Ideal for protein fibers, they bond through acidic conditions.
- Basic Dyes: Effective on acrylic fibers, they thrive in basic conditions.
- Direct Dyes: Apply directly to fibers without the need for a mordant.
- Disperse Dyes: Ideal for synthetic fibers like polyester, they require high temperatures.
- Reactive Dyes: Form strong covalent bonds with fibers, often used for cotton.
- Vat Dyes: Applied in a reduced, water-soluble form and later oxidized to become insoluble.
- Sulfur Dyes: Common for dark colors on cotton, forming insoluble compounds.
- Pigment Dyes: Particles adhere to the fabric surface without chemical bonding.
Types of dyes are an essential aspect of the textile and fashion industry. Dyes are used to add color to fabrics, yarns, and fibers. They also dye other materials like leather, paper, and food.
Understanding the different types of dyes and their properties is crucial to achieving the desired result in dyeing.
Dyes can be classified into two broad categories: natural and synthetic. As the name suggests, natural dyes are derived from natural sources like plants, animals, and minerals. On the other hand, synthetic dyes are created in a laboratory and made from chemicals.
This article will explore the different types of dyes , their properties, and their applications. We will also discuss the chemistry of dyes, the environmental and health impact, and the different dyeing techniques used in the industry.
What Are Dyes?
Dyes impart color to various materials, such as textiles, paper, and leather. They give various colors to different materials, which are not easily altered by washing, heat, light, or other factors.

Dyes differ from pigments because they chemically bond with the substrate they are applied to, whereas pigments do not. There are many different types of dyes, each with unique properties, chemical structures, and uses. Some common types of dyes include:
- Reactive dyes : They react with the fibers of the material they are applied to, forming a covalent bond. It results in a strong, permanent color resistant to washing and fading.
- Direct dyes: are water-soluble and can be applied directly to the material without needing a mordant. They are commonly used to dye cotton and other cellulose fibers .
- Acid dyes : These are used to color protein fibers such as wool, silk, and nylon. They are water-soluble and require an acidic environment to bond with the fibers.
- Vat dyes are insoluble in water and require a reducing agent to become soluble. They are commonly used to dye cotton and other cellulose fibers.
RELATED: What Is Tricot Fabric? Everything You Need To Know About Tricot Fabric
What Are The Different Types Of Natural Dyes?
Natural dyes are derived from natural sources such as plants, lichens, insects, and snails. They have been used for centuries to color textiles and are still used today because of their eco-friendliness and the unique color variations they produce.
Natural dyes can be categorized into plant-based dyes , animal-based dyes, and mineral-based dyes.
Plant-Based Dyes
Plant-based dyes are the most common types of natural dyes. They are extracted from various parts of plants, such as roots, stems, seeds, bark, leaves, and wood. Some of the most popular plant-based dyes include:
- Logwood : produces shades of purple, blue, and gray
- Alum : produces shades of yellow, green, and blue
- Woad : produces shades of blue
- Saffron : produces shades of yellow
- Madder : produces shades of red, pink, and orange
Animal-Based Dyes
Animal-based dyes are derived from various animals, such as insects and snails. They are less common than plant-based dyes but produce unique and vibrant colors . Some of the most popular animal-based dyes include:
- Cochineal : produces shades of red and pink
- Tyrian Purple : produces shades of purple
- Murex: produces shades of purple and blue
Mineral-Based Dyes
Mineral-based dyes are derived from minerals such as lead and alizarin. They are less common than plant-based and animal-based dyes but produce long-lasting and vibrant colors. Some of the most popular mineral-based dyes include:
- Lead: produces shades of white and yellow
- Alizarin: produces shades of red and orange

What Are The Different Types Of Synthetic Dyes?
Synthetic dyes are produced from various chemicals. They are widely used in the textile, leather, and paper industries. Synthetic dyes are preferred over natural dyes because of their superior cost, optical properties, and resilience.
There are different types of synthetic dyes , and each type has its unique properties and applications. The different types of synthetic dyes are given below.
- Azo dyes are the most common type of synthetic dyes. They are widely used in the textile industry to dye cotton, wool, and silk. Azo dyes are characterized by a nitrogen-nitrogen double bond (N=N) in their chemical structure.
- This double bond is responsible for azo dyes’ bright and vibrant colors. Azo dyes are also known for their excellent lightfastness and wash fastness. Some common examples of azo dyes are Acid Orange 7, Direct Red 81, and Disperse Yellow 7.
Anthraquinone Dyes
- Anthraquinone dyes are another type of synthetic dyes. They are used to dye cellulose and protein fibers. Anthraquinone dyes are characterized by an anthracene ring system in their chemical structure. This ring system is responsible for anthraquinone dyes’ bright and intense colors.
- Anthraquinone dyes are also known for their excellent lightfastness and wash fastness. Some common examples of anthraquinone dyes are Alizarin Red S, Disperse Blue 3, and Acid Blue 45.
Aniline Dyes
- Aniline dyes are a type of synthetic dyes that are used to dye silk , wool, and leather. Aniline dyes are characterized by an aniline ring in their chemical structure. This ring system is responsible for aniline dyes’ bright and vivid colors .
- Aniline dyes are also known for their excellent lightfastness and wash fastness. Some common examples of aniline dyes are Acid Green 25, Basic Red 1, and Direct Black 22.
How To Classify Dyes?
- Acid dyes are water-soluble dyes applied to wool, silk, and nylon fibers . They are typically used for dyeing protein fibers and are known for their bright, vibrant colors. Acid dyes are also used in the production of food coloring .
- Basic dyes are water-soluble dyes that color paper , leather, and textiles. They are known for their bright, intense colors and are often used in producing fluorescent dyes.
Direct Dyes
- Direct dyes are water-soluble dyes that color cotton, rayon, and other cellulose fibers. They are known for their bright, vibrant colors and are often used in the production of tie-dye clothing .
- Vat dyes are insoluble in water and are used to color cotton , wool, and other fibers. They are known for their excellent colorfastness and are often used in the production of denim clothing .
Reactive Dyes
- Reactive dyes are water-soluble dyes that color cotton, wool, and other fibers. They are known for their excellent colorfastness and are often used in producing athletic wear .
Disperse Dyes
- Disperse dyes are insoluble in water and are used to color synthetic fibers such as polyester and nylon. They are known for their excellent colorfastness and are often used in the production of outdoor clothing.
Mordant Dyes
- Mordant dyes are water-soluble dyes applied to wool, silk, and cotton fibers. They require a mordant, which helps the dye bond to the fiber. Mordant dyes are known for their excellent colorfastness and are often used to produce tapestries and other decorative textiles .
Sulfur Dyes
- Sulfur dyes are insoluble in water and are used to color cotton, rayon, and other cellulose fibers. They are known for their excellent colorfastness and are often used in the production of denim clothing .
RELATED: What Is Dri-FIT Material?
What Are The Different Types Of Dyeing Techniques?
When it comes to dyeing textiles, various techniques can be used. The technique chosen will depend on factors such as the type of material being dyed, the desired color, and the equipment available.
Aqueous Solution Dyeing
- Aqueous solution dyeing is the most common technique used for dyeing textiles. This method dissolves the dye in water to create an aqueous solution . The textile material is then immersed in the solution, and the dye is absorbed into the fibers through diffusion.
Heat Dyeing
- Heat dyeing is a technique that involves applying heat to the textile material during the dyeing process. This technique can be used to dye both natural and synthetic fibers.
- Heat is used to increase the solubility of the dye, allowing it to penetrate the fibers more easily. The temperature will depend on the type of material being dyed and the dye being used.
Ammonia Dyeing
- Ammonia dyeing is a technique that involves using ammonia to create a more alkaline environment for the dyeing process. This technique is commonly used for dyeing synthetic fibers such as polyester and nylon.
- The ammonia helps open the fibers, allowing the dye to penetrate more easily. However, this technique can be hazardous if not done properly, as ammonia can be toxic.
What Are The Different Types Of Dyeing Materials?
Dyeing cotton.
- Cotton is a popular material for dyeing due to its absorbent nature. It can be dyed with various dyes, including vat dyes and modern synthetic reactive and direct dyes. However, cotton is known to shrink during the dyeing process, so it is important to pre-wash the fabric to avoid any unwanted shrinking.
Dyeing Wool
- Wool is a protein fiber and is typically dyed with acid dyes. These dyes are known for their ability to produce vibrant colors on wool . However, wool is also prone to felting, so handling it with care during the dyeing process is essential.
Dyeing Silk
- Silk is a delicate and luxurious material that can be dyed with acid or natural dyes. However, it is essential to note that silk is sensitive to high temperatures, so it should be dyed at a lower temperature to avoid damage.
Dyeing Nylon
- Nylon is a synthetic fiber that can be dyed with acid dyes or basic dyes. Basic dyes are known for producing brighter colors on nylon, but they are also more challenging to work with than acid dyes.
Dyeing Polyester
- Polyester is a synthetic fiber that can be dyed with dispersed dyes. These dyes are specifically designed for use on polyester and are known for producing vibrant colors. However, polyester is also sensitive to high temperatures, so it should be dyed at a lower temperature to avoid damage.
Dyeing Leather
- Leather can be dyed using a variety of dyes, including acid dyes and oil-based dyes. However, the type of dye used will depend on the type of leather and the desired outcome.
Dyeing Cellulosic Fibers
- Cellulosic fibers , such as viscose and jute, can be dyed using various dye types, including vat and reactive dyes. However, it is essential to note that cellulosic fibers are sensitive to high temperatures, so they should be dyed at a lower temperature to avoid damage.
Dyeing Acrylic
- Acrylic fibers are typically dyed with basic dyes, known for producing bright colors on acrylic. However, it is essential to note that acrylic is sensitive to high temperatures, so it should be dyed at a lower temperature to avoid damage.

What Is The Chemistry Behind Dyes?
Organic compounds known as dyes impart color to a wide range of substrates, including cosmetics, papers, fur, hair, drugs, leather, waxes, textiles, plastics, and greases.
The color of dyes is due to their chromophore groups, responsible for absorbing light in the visible region of the electromagnetic spectrum.
The auxochrome groups are responsible for increasing the solubility of dyes in water and improving their affinity for the substrate. Dyes are classified based on their chemical structure and the type of substrate they are applied to.
Water-soluble dyes include reactive dyes, direct dyes, and acid dyes, while water-insoluble dyes include vat dyes and sulfur dyes. The chemical structure of dyes consists of a chromophore group and an auxochrome group.
The chromophore group is responsible for the color of the dye, while the auxochrome group enhances the color and solubility of the dye.
The chromophore group can be a nitro, azo, carbonyl, or quinone group, while the auxochrome group can be a carboxylic acid, hydroxyl, or amino group.
Dyes are applied to the substrate by forming a covalent bond with the fibers of the substrate. The interaction between the auxochrome group of the dye and the functional groups present on the surface of the substrate forms this bond.
The type of bond formed depends on the type of dye and the substrate.
Alkali, iron, and other mordants are used to improve the color fastness of dyes. Anionic dyes are used for dyeing protein fibers such as wool and silk, while cationic dyes are used for dyeing synthetic fibers such as polyester and nylon .
Environmental And Health Impact Of Dyes
Environmental impact.
- The production and use of dyes contribute to environmental pollution. The presence of dyes in wastewater can negatively impact aquatic ecosystems and harm aquatic life. Some dyes are also toxic and can persist in the environment long, leading to long-term pollution.
- The textile industry is a significant contributor to dye pollution. Synthetic dyes in textile production have been linked to increased chemical and biochemical oxygen demand (COD and BOD), which can reduce the oxygen levels in water bodies and harm aquatic life.
Health Impact
- The use of certain dyes has been linked to various health problems. For example, some dyes are carcinogenic, mutagenic, or toxic to the reproductive system . These dyes can be exposed through inhalation, ingestion, or skin contact.
- In addition, the production and use of dyes can lead to occupational health hazards . Workers in the dyeing and printing industries are often exposed to high levels of chemicals, which can lead to respiratory problems, skin irritation, and other health issues.
Natural Sources
- While synthetic dyes are commonly used, natural dyes sourced from plants, animals, and minerals are also available. These dyes are generally considered more environmentally friendly, as they are biodegradable and do not contain harmful chemicals.
- For example, indigo dye , derived from the indigo plant, has been used for centuries and is still used in some industries.
- However, natural dyes can be less cost-effective and water-soluble than synthetic dyes, making them less practical for large-scale production.
RELATED: Simplifying Yardage Calculator Fabric And Its Accurate Measurements
Frequently Asked Questions
What are the two main types of dyes.
The two main types of dyes are natural and synthetic dyes. Natural dyes are derived from plants, animals, and minerals, while synthetic dyes are made from chemicals.
What Are 10 Natural Dyes?
There are many natural dyes available. Some of the most popular ones include indigo, madder, cochineal, turmeric, pomegranate, onion skins, walnut hulls, chamomile, and marigold.
What Are The Properties Of Dyes?
Dyes have various properties, including colorfastness, lightfastness, wash fastness, and resistance to fading. The properties of dyes depend on their chemical composition and the type of material they are applied to.
What Are The Different Types Of Fabric Dyeing Techniques?
Several fabric dyeing techniques include direct dyeing, resist dyeing, yarn dyeing, piece dyeing, and printing. Each technique has advantages and disadvantages, and the choice of technique depends on the type of fabric, the desired effect, and the dye used.
What Are The Uses Of Dyes?
Dyes are used in various industries, including textile, paper, leather, and food. They are used to color fabrics, paper, and leather products and enhance the appearance of food products.
Dyes are also used in medical and scientific research to stain tissues and cells for microscopic examination.
- Recent Posts
- Understanding Knitting Yarn Types – Everything You Need To Know - March 4, 2024
- Does Velcro Stick To Felt? Exploring The Bonding Compatibility - February 26, 2024
- Is Twill Good For Summer? Exploring Warm Weather Fabric Choices - February 26, 2024
Terms and Conditions - Privacy Policy
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Dyes and Pigments: Their Structure and Properties

Dyes and pigments are the most important colorants used to add a color or to change the color of something. They are widely used in the textile, pharmaceutical , food, cosmetics, plastics, paint, ink, photographic and paper industries. Dyes are colored substances which are soluble or go into solution during the application process and impart color by selective absorption of light. Pigments are colored, colorless, or fluorescent particulate organic or inorganic finely divided solids which are usually insoluble in, and essentially chemically unaffected by, the vehicle or medium in which they are incorporated. On the other hand, the color, which is highly dependent on the chemical and physical properties of a matter, is a result of the interaction between light and substance. This chapter is focused on the chemical and structural properties of dyes and pigments, as well as the relationship between light and color.
Related papers
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Color Research & Application, 2019
This paper review is recommended for undergraduate students, graduate students, chemists, dyestuffs and colorists. They will find it interesting, informative and very readable. Several multi choice problems and their solutions in the basics and fundamentals of colors, dyes and pigments chemistry were represented in this paper review. Reviewing the basic information, fundamental understanding, principles and/or the knowledge of colors, dyes and pigments chemistry via multi choice problems and their solutions is interesting type of reviewing and can be used as an excellent tool for measuring and/or test the deep understanding of undergraduate and graduate students for colors, dyes and pigments chemistry. The paper includes, contains and/or covers topics like, the relation between color and constitution and/or Witt's theory, fibers, definitions for dyes and pigments and other definitions and vital informations in color, dyes and pigments chemistry. The paper also involve synthesis, properties, classifications and uses of many aromatic and/or heterocyclic dyes. Such dyes, like nitro dyes, nitroso dyes, azo dyes, diarylmethane dyes, triarylmethane dyes, anthraquinone dyes, cyanine dyes, azine dyes, phthaleine dyes, indigoid dyes, acridine dyes, xanthen dyes and phthalocyanine dyes. This paper review acts as a mordant and/or stabilizers for some of the basic informations, principles and/or the knowledge in colors, dyes and pigments chemistry. It is valuable both for getting an overview in the field of color, dyes and pigments chemistry and as a mine of information for the dyestuff chemists. In addition, this paper review can be used and/or will be most valuable in domestic and/or international chemistry competitions in organic chemistry as general and particularly, in colors, dyes and pigments chemistry. Besides, this paper review can be printed and used as a thesis and/or as a note book students learning and lectures. Also, it is can be used in students examinations tests in chemistry departments and/or chemistry institutions of any domestic and/or international university.
The Chemical Educator, 1997
Pigments Pigments A pigment is a material like metal oxides, azo-dye etc. that changes the colour of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light. It must be stable in solid form at ambient temperatures. Q: Give one example of each white, black, green, purple, blue and red pigment. Purple pigments Cobalt Violet: Cobaltous orthophosphate. Manganese violet: NH 4 MnP 2 O 7 Manganic ammonium pyrophosphate Blue pigment Ultramarine: pigment of sulfur-containing sodio-silicate (Na 8-10 Al 6 Si 6 O 24 S 2-4) Cobalt Blue: cobalt(II) stannate Prussian Blue: a synthetic pigment of ferric hexacyanoferrate (Fe 7 (CN) 18). Green pigment Chrome green : chromic oxide (Cr 2 O 3) Paris Green: cupric acetoarsenite (Cu(C 2 H 3 O 2) 2 •3Cu(AsO 2) 2) Yellow pigment Cadmium Yellow: cadmium sulfide (CdS) Chrome Yellow: natural pigment of plumbous chromate (PbCrO 4). Mosaic gold: stannic sulfide (SnS 2) Orange pigment Chrome Orange: a naturally occurring pigment (PbCrO 4 + PbO) Red pigments Red Ochre: anhydrous Fe 2 O 3 Red Lead: lead tetroxide, Pb 3 O 4 Vermilion: Occurs naturally in mineral cinnabar. Mercuric sulfide (HgS) Black pigments Carbon Black Lamp Black White pigment Titanium White: titanic oxide (TiO 2
Encyclopedia of Polymer Science and Technology, 2002
Predigt zum Dreifaltigkeitsfest, 2024
Linköping electronic conference proceedings, 2024
Anuario Colombiano de Historia Social y de la Cultura, 2014
Automobili con motore a 16 cilindri, 1994
ACTA TECHNICA CORVINIENSIS – Bulletin of Engineering [e-ISSN: 2067-3809] TOME XIII [2020] | FASCICULE 2 [April – June] , 2020
Textos para Discussão, 2019
Brownstone Journal, 2024
BMC complementary medicine and therapies, 2022
Social science review archive , 2024
Politische Vierteljahresschrift, 2006
Acta Tropica, 2010
Nanda Fajrianti Arifin, 2024
European Spine Journal, 2010
Hacettepe üniversitesi sağlık bilimleri fakültesi dergisi, 2017
Professional Book Research in Organizations and Management Publishing, Bradford Special Issue, 2022., 2022
Journal of the Neurological Sciences, 2017
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Unlock the Secrets: A Deep Dive into the Classification of Dyes and Pigments

Until the discovery of synthetic dyes and pigments, natural colorants such as flowers, plants, and minerals were the only sources of natural colorants. There has been a massive increase in the number and kind of colorants, making their classification mandatory. In this guide, we will discuss the classification of dyes and pigments.
What are dyes and pigments? How they are produced? And what are the classifications of dyes and pigments? Dyes or colorants are categorized according to various factors, like structure, source, color, solubility, and application.
They are divided into two categories: dyes and pigments based on the chemical structure and application method of dyes. Azo dyes are classified as anthraquinone, anthraquinone-containing dyes, indigo dyes, phthalocyanine dyes, sulfur dyes, nitro dyes, and nitroso dyes based on the chemical structure of the dyes.
Based on the application method, pigments were grouped as reactive, dispersed, acid, basic, direct, and vat dyes. Nonetheless, based on their chemical composition, pigments can also be considered organic versus inorganic.
So, let’s dive right into the classification of dyes and pigments and understand their significance in various industries.
What are dyes and pigments?
Dyes and pigments are both substances that impart color to different materials, although they work differently and use different materials, they can color the same things:
1. Coloring mechanism
Dyes can be absorbed into the material they are applied to, while pigments can be used to the surface.
2. Solubility
Dyes are water-soluble or organic solvents, whereas pigments are insoluble in water.
3. Particle size
Dyes are quite better and finer compared to pigments.
4. UV stability
Dyes are not UV stable, whereas pigments show good UV stability.
Dyes impart more vibrant, brighter colors than pigments.
6. Materials colored
Dyes are widely used to color food, drugs, cosmetics, textiles, paper, and leather, while pigments are used to color any polymeric substrate and skin care products.
Types of Dyes
Dyes are classified as natural dyes and synthetic dyes. Natural dyes are those dyes or colorants derived from natural sources, such as green vegetables, plants, trees, animals, and minerals. On the other hand, synthetic dyes are colors or dyes produced from coal tar or petrochemicals.
There has been a long debate between natural food colors vs. synthetic food colors , making them different from each other. Both dyes have their own pros and cons and different areas of application in various industries. In addition, there are various types of dyes, including:
1. Direct Dyes
Direct dyes are those dyes applied directly to the material without requiring a mordant. They are widely used to color cellulosic fibers, such as rayon, cotton, and viscose.
2. Reactive Dyes
As the name suggests, these dyes are chemically reactive and create a covalent bond with the fibers, which shows exceptional color retention and wash fastness. Reactive dyes are best known for dyeing natural fibers like silk, wool, and cotton.
3. Disperse Dyes
These dyes are used to color hydrophobic materials, like nylon and polyester. Disperse dyes require extreme temperatures and are dispersed into fine particles to dye the materials effectively.
4. Acid Dyes
Acid dyes provide good light fastness and a wide range of colors used to dye protein fibers, such as silk and wool.
5. Basic Dyes
These are colorants perfect for modified acrylic fibers, certain protein fibers, and acrylic fibers.
6. Vat Dyes
These dyes are used to color cellulosic fibers and require a reducing agent to solubilize them before the coloring process.
Types of Pigments
Here are the following types of pigments, including:
1. Inorganic Pigments
These pigments offer excellent light fastness and chemical stability and are derived from minerals, including titanium dioxide (a white pigment) and iron oxides (yellow, red, and brown hues).
2. Organic Pigments
Organic pigments, known as synthetic dye colorants are derived from carbon-based compounds. Now you might be wondering what are synthetic dyes and what are their importance in a particular industry. They offer a wide range of colors and are best known for their excellent versatility.
3. Pearlescent Pigments
These pigments offer a shimmering effect and are widely used in plastics, cosmetics, and automotive coatings.
4. Fluorescent Pigments
Fluorescent pigments are used to provide more vibrant, bright colors by absorbing light at specific wavelengths and emitting light at longer wavelengths. These pigments are most commonly utilized in safety and novelty products.
5. Phosphorescent Pigments
These pigments store light energy and release it gradually over time, thus creating the famous glow-in-the-dark effect.
In the bottom line, it can be concluded that both dyes and pigments are widely used in a variety of applications, ranging from cosmetics, foods, pharmaceuticals, plastics, textiles, paper, printing, and more. Based on their areas of applications, chemical properties, and resistance to light, they are broadly classified and are used for various uses.
Both dyes and pigments are regulated and monitored by the U.S. FDA and EFSA to ensure the safety and proper use of these dyes or pigments. If you are a business or individual professional looking for high-quality dyes and pigments for your business or industrial applications, look no further than Hridhan Chem Pvt. Ltd.
Hridhan Chem is a reputed manufacturer and exporter of premium quality dyes and pigments that can be used in different industry verticals, including foods, pharmaceuticals, and cosmetics. For more information on a wide range of synthetic dyes and pigments we produce at our manufacturing facility, get in touch with us today!
1 What are the classifications of dyes? Dyes are classified on the basis of their solubility, application, and chemical composition, such as direct dyes, acid dyes, basic dyes, vat dyes, disperse dyes, sulfur dyes, reactive dyes, and more.
2 What are dyes and pigments? Dyes and pigments refer to substances that are widely used for coloring different materials they are applied to. Particle size is the main chemical property that makes dyes distinct from pigments. Furthermore, dyes offer an exceptional fine tone compared to pigments.
3 What are the classifications of pigment dyes? Pigments are classified, depending on how they are applied. They are further grouped as basic, vat, direct, dispersed, and reactive dyes. Nevertheless, the classification of organic and inorganic pigments is done properly.
Related posts

Synthetic Dyes Explained: Revolutionizing Color in Industry

Synthetic dyes used in home and personal care products
What are Dyes: Understanding Different Types, Uses, and Properties
Exploring the World of Basic Dyes
Colors play a fundamental role in our lives, adding vibrancy and character to everything around us. From the clothes we wear to the products we use, colors evoke emotions, convey information, and leave lasting impressions. In the realm of dyes and pigments, basic dyes are a fascinating class that has contributed significantly to the world of textiles, cosmetics, and even biology. In this blog, we will delve into the basics of basic dyes, exploring their uses, importance, chemistry, and more.
Understanding Basic Dyes
Basic dyes are a type of synthetic dyes distinguished by their strong attraction to cationic or positively charged materials. They are widely used to color textiles, paper, leather, and other materials. Basic dyes are so named because they are primarily composed of basic or alkaline molecules. These dyes are typically water-soluble and can readily form a bond with materials that have a negative charge, such as cellulosic fibers.
Key Characteristics of Basic Dyes
1. Positively Charged: Basic dyes carry a positive charge, making them suitable for dyeing materials with a negative charge.
2. Bright and Vivid Colors: Basic dyes are known for producing bright and vibrant colors, making them popular choices for coloring textiles, paper, and cosmetics.
3. Water-Soluble: Most basic dyes are water-soluble, allowing for easy application in dyeing processes.
Uses of Basic Dyes
1. Textile Industry : One of the primary applications of basic dyes is in the textile industry. They are used to color natural fibers like cotton, wool, and silk, producing a wide range of colors. The vibrant and long-lasting hues achieved with basic dyes make them indispensable in the fashion and apparel sectors.
2. Paper and Printing: Basic dyes are used in the paper industry to color paper and cardboard products. They are also employed in the printing industry for producing vivid and sharp images in newspapers, magazines, and packaging materials.
3. Leather Coloring: Basic dyes find application in the leather industry to color leather products such as shoes, bags, and upholstery.
4. Cosmetics: Basic dyes are used in cosmetics, especially in the production of lipsticks, nail polishes, and hair dyes. Their ability to produce intense colors makes them popular choices for enhancing personal beauty products.
5. Biological Staining: In biology and microscopy, basic dyes are employed for staining tissues and cells. Examples include hematoxylin and eosin, which are used to stain various cellular components for microscopic examination.
Importance of Basic Dyes
1. Color Diversity: Basic dyes offer a wide spectrum of colors, allowing for a diverse range of applications in various industries. This diversity is crucial for meeting consumer preferences and industry demands.
2. Cost-Effective: Basic dyes are often more cost-effective than some other types of dyes, making them a preferred choice for industries that require large-scale coloration.
3. Long-Lasting Colors: Basic dyes are known for their excellent colorfastness, ensuring that the colors remain vibrant and stable over time. This is particularly important in textiles and cosmetics, where color durability is a key factor.
4. Compatibility with Natural Fibers: Basic dyes have a natural affinity for cellulosic fibers like cotton, making them ideal for coloring fabrics made from these materials.
5. Biological Research: In the field of biology, basic dyes play a crucial role in staining tissues and cells, aiding researchers in visualizing and studying biological specimens.
Challenges and Considerations
While basic dyes offer many advantages, they are not without their challenges and considerations:
1. Limited Application Range: Basic dyes are best suited for dyeing materials with a negative charge. They are less effective on synthetic fibers and materials with neutral or positive charges.
2. Environmental Impact: The dyeing process with basic dyes may involve the use of chemicals and large quantities of water, contributing to environmental concerns if not managed properly.
3. Health and Safety: Some basic dyes may contain chemicals that can be harmful if not handled with care. Proper safety measures must be observed during their production and use.
Basic dyes are a remarkable class of synthetic dyes that have left a colorful imprint on various industries, from fashion to biology. Their positive charge and ability to produce vibrant and long-lasting colors have made them indispensable in textile, paper, leather, and cosmetic applications. While basic dyes come with certain challenges and environmental considerations, their importance in adding life and character to our everyday products cannot be understated. As technology advances and sustainability becomes a key concern, we can expect continued innovation in the field of basic dyes to meet the evolving needs of industries and consumers alike.
Related Post
Exploring the dichotomy: cationic dyes vs. anionic dyes for paper, unveiling the best dye for the paper industry: a comprehensive guide, why are eco-friendly dyes trending in the global markets.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Eco-Friendly Textile Dyeing and Finishing
Textile Dyes: Dyeing Process and Environmental Impact
Submitted: 20 March 2012 Published: 16 January 2013
DOI: 10.5772/53659
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Eco-Friendly Textile Dyeing and Finishing
Edited by Melih Günay
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
23,172 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Overall attention for this chapters
Author Information
Farah maria drumond chequer.
- USP, Department of Clinical, Toxicological and Bromatological Analyses, Faculty of Pharmaceutical Sciences at Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil
Gisele Augusto Rodrigues de Oliveira
Elisa raquel anastácio ferraz, juliano carvalho cardoso.
- UNESP, Department of Analytical Chemistry, Institute of Chemistry at Araraquara, State of São Paulo University Júlio de Mesquita Filho, Araraquara, SP, Brazil
Maria Valnice Boldrin Zanoni
Danielle palma de oliveira.
*Address all correspondence to:
1. Introduction
Dyes may be defined as substances that, when applied to a substrate provide color by a process that alters, at least temporarily, any crystal structure of the colored substances [ 1 , 2 ]. Such substances with considerable coloring capacity are widely employed in the textile, pharmaceutical, food, cosmetics, plastics, photographic and paper industries [ 3 , 4 ]. The dyes can adhere to compatible surfaces by solution, by forming covalent bond or complexes with salts or metals, by physical adsorption or by mechanical retention [ 1 , 2 ]. Dyes are classified according to their application and chemical structure, and are composed of a group of atoms known as chromophores, responsible for the dye color. These chromophore-containing centers are based on diverse functional groups, such as azo, anthraquinone, methine, nitro, arilmethane, carbonyl and others. In addition, electrons withdrawing or donating substituents so as to generate or intensify the color of the chromophores are denominated as auxochromes. The most common auxochromes are amine, carboxyl, sulfonate and hydroxyl [ 5 - 7 ].
It is estimated that over 10,000 different dyes and pigments are used industrially and over 7 x 10 5 tons of synthetic dyes are annually produced worldwide [ 3 , 8 , 9 ]. Textile materials can be dyed using batch, continuous or semi-continuous processes. The kind of process used depends on many characteristics including type of material as such fiber, yarn, fabric, fabric construction and garment, as also the generic type of fiber, size of dye lots and quality requirements in the dyed fabric. Among these processes, the batch process is the most common method used to dye textile materials [ 10 ].
In the textile industry, up to 200,000 tons of these dyes are lost to effluents every year during the dyeing and finishing operations, due to the inefficiency of the dyeing process [ 9 ]. Unfortunately, most of these dyes escape conventional wastewater treatment processes and persist in the environment as a result of their high stability to light, temperature, water, detergents, chemicals, soap and other parameters such as bleach and perspiration [ 11 ]. In addition, anti-microbial agents resistant to biological degradation are frequently used in the manufacture of textiles, particularly for natural fibers such as cotton [ 11 , 12 ]. The synthetic origin and complex aromatic structure of these agents make them more recalcitrant to biodegradation [ 13 , 14 ]. However, environmental legislation obliges industries to eliminate color from their dye-containing effluents, before disposal into water bodies [ 9 , 12 ].
The textile industry consumes a substantial amount of water in its manufacturing processes used mainly in the dyeing and finishing operations of the plants. The wastewater from textile plants is classified as the most polluting of all the industrial sectors, considering the volume generated as well as the effluent composition [ 15 - 17 ]. In addition, the increased demand for textile products and the proportional increase in their production, and the use of synthetic dyes have together contributed to dye wastewater becoming one of the substantial sources of severe pollution problems in current times [ 6 , 9 ].
Textile wastewaters are characterized by extreme fluctuations in many parameters such as chemical oxygen demand (COD), biochemical oxygen demand (BOD), pH, color and salinity. The composition of the wastewater will depend on the different organic-based compounds, chemicals and dyes used in the dry and wet-processing steps [ 6 , 18 ]. Recalcitrant organic, colored, toxicant, surfactant and chlorinated compounds and salts are the main pollutants in textile effluents [ 17 ].
In addition, the effects caused by other pollutants in textile wastewater, and the presence of very small amounts of dyes (<1 mg/L for some dyes) in the water, which are nevertheless highly visible, seriously affects the aesthetic quality and transparency of water bodies such as lakes, rivers and others, leading to damage to the aquatic environment [ 19 , 20 ].
During the dyeing process it has been estimated that the losses of colorants to the environment can reach 10–50% [ 13 , 14 , 17 , 21 , 22 ]. It is noteworthy that some dyes are highly toxic and mutagenic, and also decrease light penetration and photosynthetic activity, causing oxygen deficiency and limiting downstream beneficial uses such as recreation, drinking water and irrigation [ 13 , 14 , 23 ]
With respect to the number and production volumes, azo dyes are the largest group of colorants, constituting 60-70% of all organic dyes produced in the world [ 2 , 24 ]. The success of azo dyes is due to the their ease and cost effectiveness for synthesis as compared to natural dyes, and also their great structural diversity, high molar extinction coefficient, and medium-to-high fastness properties in relation to light as well as to wetness [ 2 , 25 ]. They have a wide range of applications in the textile, pharmaceutical and cosmetic industries, and are also used in food, paper, leather and paints [ 26 , 27 ]. However, some azo dyes can show toxic effects, especially carcinogenic and mutagenic events [ 27 , 28 ].
The toxic effects of the azo dyes may result from the direct action of the agent itself or of the aryl amine derivatives generated during reductive biotransformation of the azo bond [ 22 ]. The azo dyes entering the body by ingestion can be metabolized to aromatic amines by the azoreductases of intestinal microorganisms. If the dyes are nitro, they can be metabolized by the nitroredutases produced by the same microorganisms [ 29 ]. Mammalian liver enzymes and other organizations may also catalyze the reductive cleavage of the azo bond and the nitroreduction of the nitro group. In both cases, if N-hydroxylamines are formed, these compounds are capable of causing DNA damage [ 29 , 30 ].
One of the most difficult tasks confronted by the wastewater treatment plants of textile industries is the removal of the color of these compounds, mainly because dyes and pigments are designed to resist biodegradation, such that they remain in the environment for a long period of time. For example, the half-life of the hydrolyzed dye Reactive Blue 19 is about 46 years at pH 7 and 25°C [ 31 , 32 ].
Carneiro et al. (2010) designed and optimized an accurate and sensitive analytical method for monitoring the dyes C.I. Disperse Blue 373 (DB373), C.I. Disperse Orange 37 (DO37) and C.I. Disperse Violet 93 (DV93) in environmental samples. This investigation showed that DB373, DO37 and DV93 were present in both untreated river water and drinking water, indicating that the effluent treatment (pre-chlorination, flocculation, coagulation and flotation) generally used by drinking water treatment plants, was not entirely effective in removing these dyes. This study was confirmed by the mutagenic activity detected in these wastewaters [ 33 ].
In this context, and considering the importance of colored products in present day societies, it is of relevance to optimize the coloring process with the objective of reducing the environmental impact of the textile industry. For this purpose, liposomes could be used to carry several encapsulated dyes, and hence improve the mechanical properties of textile products, resulting in better wash fastness properties and reducing the process temperature, thus economizing energy [ 34 ]. Another way is to use ultrasonic energy, studied with the objectives of improving dye productivity and washing fastness, and reducing both energy costs and water consumption [ 35 ].
Considering the fact that the textile dyeing process is recognized as one of the most environmentally unfriendly industrial processes, it is of extreme importance to understand the critical points of the dyeing process so as to find alternative, eco-friendly methods.
2. Dyeing process
The dyeing process is one of the key factors in the successful trading of textile products. In addition to the design and beautiful color, the consumer usually looks for some basic product characteristics, such as good fixation with respect to light, perspiration and washing, both initially and after prolonged use. To ensure these properties, the substances that give color to the fiber must show high affinity, uniform color, resistance to fading, and be economically feasible [ 36 ].
Modern dyeing technology consists of several steps selected according to the nature of the fiber and properties of the dyes and pigments for use in fabrics, such as chemical structure, classification, commercial availability, fixing properties compatible with the target material to be dyed, economic considerations and many others [ 36 ].
Dyeing methods have not changed much with time. Basically water is used to clean, dye and apply auxiliary chemicals to the fabrics, and also to rinse the treated fibers or fabrics [ 37 ]. The dyeing process involves three steps: preparation, dyeing and finishing, as follows:
Preparation is the step in which unwanted impurities are removed from the fabrics before dyeing. This can be carried out by cleaning with aqueous alkaline substances and detergents or by applying enzymes. Many fabrics are bleached with hydrogen peroxide or chlorine-containing compounds in order to remove their natural color, and if the fabric is to be sold white and not dyed, optical brightening agents are added [ 37 ].
Dyeing is the aqueous application of color to the textile substrates, mainly using synthetic organic dyes and frequently at elevated temperatures and pressures in some of the steps [ 37 , 38 ]. It is important to point out that there is no dye which dyes all existing fibers and no fiber which can be dyed by all known dyes [ 39 ]. During this step, the dyes and chemical aids such as surfactants, acids, alkali/bases, electrolytes, carriers, leveling agents, promoting agents, chelating agents, emulsifying oils, softening agents etc [ 23 , 38 ] are applied to the textile to get a uniform depth of color with the color fastness properties suitable for the end use of the fabric [ 37 ].This process includes diffusion of the dye into the liquid phase followed by adsorption onto the outer surface of the fibers, and finally diffusion and adsorption on the inner surface of the fibers [ 40 ]. Depending on the expected end use of the fabrics, different fastness properties may be required. For instance, swimsuits must not bleed in water and automotive fabrics should not fade after prolonged exposure to sunlight [ 37 ]. Different types of dye and chemical additives are used to obtain these properties, which is carried out during the finishing step. Dyeing can also be accomplished by applying pigments (pigments differ from dyes by not showing chemical or physical affinity for the fibers) together with binders (polymers which fix the pigment to the fibers) [ 39 , 41 ].
Finishing involves treatments with chemical compounds aimed at improving the quality of the fabric. Permanent press treatments, water proofing, softening, antistatic protection, soil resistance, stain release and microbial/fungal protection are all examples of fabric treatments applied in the finishing process [ 37 ].
Dyeing can be carried out as a continuous or batch process [ 37 ]. The most appropriate process to use depends on several factors, such as type of material (fiber, yarn, fabric, fabric construction, garment), generic type of fiber, size of dye batch and quality requirements for the dyed fabric, but batch processes are more commonly used to dye textile materials [ 10 ].
In continuous processing, heat and steam are applied to long rolls of fabric as they pass through a series of concentrated chemical solutions. The fabric retains the greater part of the chemicals while rinsing removes most of the preparation chemicals. Each time a fabric is passed through a solution, an amount of water equivalent to the weight of the fabric must be used [ 37 ].
In batch processing, sometimes called exhaust dyeing, since the dye is gradually transferred from the dye bath to the material being dyed over a relatively long period of time [ 10 ], the dyeing occurs in the presence of dilute chemicals in a closed equipment such as a kier, kettle, beam, jet or beck [ 37 ]. Unlike the continuous process, instead of being passed through various baths in a long series of equipment sections, in the batch process the fabric remains in a single piece of equipment, which is alternately filled with water and then drained, at each step of the process. Each time the fabric is exposed to a separate bath, it uses five to ten times its own weight in water [ 37 ].
Some batch dyeing machines only operate at temperatures up to 100ºC. However, the system can be pressurized, allowing for the use of temperatures above 100ºC. Cotton, rayon, nylon, wool and some other fibers dye well at temperatures of 100ºC or below. Polyester and some other synthetic fibers dye more easily at temperatures above 100ºC [ 10 ].
Since the degree of dye fixation depends on the nature of the fiber, it is important to consider this topic. The fibers used in the textile industry can be divided into two main groups denominated natural fibers and synthetic fibers [ 36 , 37 ]. Natural fibers are derived from the environment (plants or animals), such as wool, cotton, flax, silk, jute, hemp and sisal, most of which are based on cellulose and proteins. On the other hand, synthetic fibers are organic polymers, mostly derived from petroleum sources, for example, polyester, polyamide, rayon, acetate and acrylic [ 37 , 39 , 42 ]. The two most important textile fibers are cotton, the largest, and polyester [ 43 , 44 ].
Cotton has been used for over 7000 years, and consists of mainly cellulose, natural waxes and proteins. The large number of hydroxyl groups on the cellulose provides a great water absorption capacity [ 39 ].
Several aromatic polyesters have been synthesized and studied. Of these, polyethylene terephthalate (PET) and polybutylene terephthalate (PBT) have been produced commercially for more than 50 years. Amongst other uses, PET has been used worldwide for the production of synthetic fibers due to its good physical properties. PET is manufactured from ethylene glycol (EG) and terephthalic acid (TPA) or dimethyl terephthalate (DMT). The polymerization proceeds in two steps: esterification and condensation reactions [ 45 ]. The polymer produced after condensation is solidified by jets of cold water and cut into regular granules which frequently have a cubic form. Then, the polymer melt is spun and the yarns are solidified by a stream of cold air [ 39 ].
The dye can be fixed to the fiber by several mechanisms, generally in aqueous solution, and may involve primarily four types of interaction: ionic, Van der Waals and hydrogen interactions, and covalent bonds [ 5 ].
Ionic interactions result from interactions between oppositely charged ions present in the dyes and fibers, such as those between the positive center of the amino groups and carboxyl groups in the fiber and ionic charges on the dye molecule, and the ionic attraction between dye cations and anionic groups (-SO 3 - and –CO 2 - ) present in the acrylic fiber polymer molecules. Typical examples of this type of interaction can be found in the dyeing of wool, silk and polyamide [ 5 , 36 , 41 ].
Van der Waals interactions come from a close approach between the π orbitals of the dye molecule and the fiber, so that the dye molecules are firmly "anchored" to the fiber by an affinity process without forming an actual bond. Typical examples of this type of interaction are found in the dyeing of wool and polyester with dyes with a high affinity for cellulose [ 36 ].
Hydrogen interactions are formed between hydrogen atoms covalently bonded in the dye and free electron pairs of donor atoms in the center of the fiber. This interaction can be found in the dyeing of wool, silk and synthetic fibers such as ethyl cellulose [ 5 , 36 ].
Covalent bonds are formed between dye molecules containing reactive groups (electrophilic groups) and nucleophilic groups on the fiber, for example, the bond between a carbon atom of the reactive dye molecule and an oxygen, nitrogen or sulfur atom of a hydroxy, amino or thiol group present in the textile fiber. This type of bond can be found in the dyeing of cotton fiber [ 5 , 36 , 46 ].
2.1. Application of liposome-based technology in textile dyeing process
There is increasing interest in the textile industry in the development of eco-friendly textile processing, in which the use of naturally occurring materials such as phospholipids, would become important [ 47 ]. Phospholipids are natural surfactants and in the presence of water, they organize themselves so as to reduce unfavorable interactions between their hydrophobic tails and the aqueous solution; their hydrophilic head groups exposed to the aqueous phase forming vesicles. Liposomes or phospholipid vesicles are featured by clearly separate hydrophilic and hydrophobic regions [ 34 , 48 ].
Liposomes were first produced in England in 1961 by Alec D. Bangham, who was studying phospholipids and blood clotting. He found that when phospholipids were added to water, they immediately formed a sphere, because one end of each molecule was water soluble, while the opposite end was water insoluble [ 49 ]. From a chemical point of view, the liposome is an amphoteric compound containing both positive and negative charges [ 34 , 50 ].
Liposomes are defined as a structure composed of lipid vesicle bilayers which can encapsulate hydrophobic or hydrophilic compounds in the lipid bilayer or in the aqueous volume, respectively [ 51 ]. These structures are usually made up of phosphatidylcholine (PC), which has a hydrophilic part consisting of phosphate and choline groups and a hydrophobic part composed of two hydrocarbon chains of variable length [ 49 , 52 , 53 ].
Liposomes are often distinguished according to their number of lamellae and size. Small unilamellar vesicles (SUV), large unilamellar vesicles (LUV) and large multilamellar vesicles (MLV) or multivesicular vesicles (MVV) can be differentiated [ 49 ]. The diameters of liposomes vary from a nanometer to a micrometer [ 34 ]. Multilamellar liposomes (MLV) usually range from 500 to 10,000 nm. Unilamellar liposomes can be small (SUV) or large (LUV); SUV are usually smaller than 50 nm and LUV are usually larger than 50 nm. Very large liposomes are called giant liposomes (10,000 - 10,00,000 nm). They can be either unilamellar or multilamellar. The liposomes containing encapsulated vesicles are called multi-vesicular and they range from 2,000-40,000 nm. LUVs with an asymmetric distribution of phospholipids in the bilayers are called asymmetric liposomes [ 54 ]. The thickness of the membrane (phospholipid bilayer) measures approximately 5 to 6 nm [ 49 ].
According to Sivasankar, Katyayani (2011), the preparation of liposomes is based on lipids, and those normally used are:
Natural phospholipids:
Phosphatidyl choline (PC) - Lecithin
Phosphatidyl ethanolamine (PE) - Cephalin
Phosphatidyl serine (PS)
Phosphatidyl inositol (PI)
Phosphatidyl glycerol (PG)
Synthetic phospholipids:
For saturated phospholipids:
Dipalmitoyl phosphatidyl choline (DPPC)
Distearoyl phosphatidyl choline (DSPC)
Dipalmitoyl phosphatidyl ethanolamine (DPPE)
Dipalmitoyl phosphatidyl serine (DPPS)
Dipalmitoyl phosphatidic acid (DPPA)
Dipalmitoyl phosphatidyl glycerol (DPPG)
For unsaturated phospholipids:
Dioleoyl phosphatidyl choline (DOPC)
Dioleoyl phosphatidyl glycerol (DOPG) [ 52 ].
Natural acidic lipids, such as PS, PG, PI, PA (phosphatidic acid) and cardiolipin (CL), are added when anionic liposomes are desired, and cholesterol is often included to stabilize the bilayer. These molecules are derivatives of glycerol with two alkyl groups and one amphoteric group [ 34 ].
Phosphatidylcholine is the biological lipid most widely used for producing liposomes. Liposomes based on phosphatidylcholine consist of phosphatidic acid and glycerin, with two alcohol groups esterified by fatty acids and a third group esterified by phosphoric acid, to which the amino alcohol choline is added as a polar group [ 34 , 49 ].
According to Barani, Montazer (2008), normally four different methods can be used for the preparation of liposomes:
Dry lipid film;
Micelle-forming detergents;
Alcohol injection technology [ 34 ].
Liposomes have two distinct roles: they can provide an excellent model for biological membranes, and they are being developed as controlled delivery systems for hydrophilic and lipophilic agents [ 34 , 53 , 55 ]. They are promising candidates for adjuvant and carrier systems for drug delivery, are well-documented, and can be used for the same purpose in textile materials [ 33 ].
Encapsulation or liposome technology is applied in numerous fields, such as in pharmaceuticals, cosmetics, foods, detergents, textiles and other applications where it is important to liberate the encapsulated material slowly [ 34 , 50 ]. This new clean technology has already been adopted by some textile industries [ 52 ]. In recent years, liposomes have been examined as a way of delivering dyes to textiles in a cost-effective and environmentally sensitive way [ 56 ].
Conventional dyeing processes consume a great deal of energy, a significant amount of which is wasted in controlling the process parameters in order to achieve uniform results. With respect to the carrier role of liposomes, they can be used in several textile processes such as textile finishing and dyeing, with several types of dyes and fibers. They are nontoxic, biodegradable, and can encapsulate a wide range of solutes [ 34 ]. In addition, the main advantages of liposomes are a clear reduction in dyeing temperature (about 10°C as compared to conventional dyeing), improved quality of the textiles produced, with additional benefits with respect to material weight yield during subsequent spinning, improved smoothness and mechanical properties of the dyed textiles, and a clear reduction in the contamination load of the dye baths [ 52 , 57 ]. Low temperature gives a more natural feel and improved quality, with lower environmental impact [ 34 ].
In recent years, liposomes have been used in the textile industry as a carrier for auxiliary materials (leveling, retarding and wetting agents) in dyeing, mainly for wool dyeing, and for finishing processes [ 34 , 55 ]. One of the most common problems with textile auxiliaries is that they fail to form a complex in the solution bath. This problem can be solved by using liposomes with selected positive or negative charges. Liposomes can be prepared according to the type of process, solute material and fiber structure [ 34 ]. Liposomes from phospholipids have been widely used as a dye carrier in the dyeing process, and create eco-friendly textile processes. Due to their structural properties, liposomes can encapsulate hydrophilic dyes (reactive, acid and basic dyes) in the aqueous phase, and hydrophobic dyes (disperse dyes) in the phospholipid bilayers [ 58 ]. Liposomes containing a dye are generally large, irregular and unilamellar [ 50 ].
According to Barani & Montazer (2008), the application of liposomes in textile processing can be useful when the release of the solute material is important, and improves the final properties of the products. A wetting agent is required in the conventional bleaching bath of cotton fabrics, but this step can be eliminated by using liposomes. The presence of liposomes in the peroxide bleaching bath can improve the mechanical properties of fabrics and their brightness. Liposomes contain particles of oxidant present in the bleaching solution that represent an unusual reservoir, and release the bleaching agent gradually into the bleaching bath. Moreover, the encapsulation of catalysts used for the decomposition of hydrogen peroxide radicals can be another factor in retarding the rate of decomposition. In this way, liposomes act as a stabilizing agent in the bleaching bath [ 34 ].
The role of auxiliary products is very important in textile dyeing with disperse dyes [ 53 ]. These compounds show extremely low solubility in water and dispersing agents are needed to maintain a fine, stable dispersion throughout the whole dyeing process at the different temperatures. Martí et al. (2007) analyzed the usefulness of commercial textile liposomes as dispersing agents, and observed that liposomes could be considered as suitable dispersing auxiliaries for polyester dyeing at high temperatures, considering their capacity to stabilize dye dispersions and achieve a suitable dye exhaustion level, with the added value of their environmentally friendly nature [ 53 ]. Liposomes clearly improve the dispersion efficiency as compared to conventional dispersing agents [ 34 ].
Additionally, liposomes for textile use show a similar price to that of synthetic surfactants used in the dyeing of polyester with disperse dyes. However, the new technology is more environmentally friendly, and hence the reduction in the environmental problem can lead to economic advantages [ 53 ]. In addition, liposome preparations tend not to foam. This is an advantage that distinguishes liposomes from other textile auxiliaries [ 34 ].
According to Martí et al. (2010), the dyeing of wool and wool blends with the aid of liposomes has demonstrated better quality, energy saving and a reduction in the environmental impact and also the temperature could be reduced, resulting in less fiber damage. Moreover, dye bath exhaustion was shown to be over 90% at the lower temperature (80°C) used, resulting in significant savings in energy costs [ 55 ]. The impact of the dyeing process on the environment was also considerably lower, the COD being reduced by about 1000 units [ 53 ].
Therefore, liposome-based technology is an alternative, eco-friendly method, which could reduce the environmental impact, offering technical and economic advantages for the textile industry.
2.2. Effect of ultrasonic energy on the dyeing process
Ultrasound-assisted textile dyeing was first reported by Sokolov and Tumansky in 1941[ 59 ]. The basic idea of this technology is that ultrasound can enhance mass transfer by reducing the stagnant cores in the yarns. The improvements observed are generally attributed to cavitation phenomena and to other resulting physical effects such as dye dispersion (breaking up of aggregates with high relative molecular mass), degassing (expulsion of dissolved or entrapped air from the fiber capillaries), strong agitation of the liquid (reduction in thickness of the fiber-liquid boundary layer), and swelling (enhancement of dye diffusion rate inside the fiber) [ 59 , 60 ].
According to Vankar & Shanker (2008), ultrasound allows for process acceleration, obtaining the same or better results than existing techniques, but under less extreme conditions, i.e., lower temperatures and lower concentrations of the chemicals used. Wet textile processes assisted by ultrasound are of great interest to the textile industry for this reason [ 61 ], and Khatri et al. (2011) showed that the dyeing of polyester fiber using ultrasonic energy resulted in an increased dye uptake and enhanced dyeing rate [ 35 ].
Due to the revolution in environmental protection, the use of ultrasonic energy as a renewable source of energy in textile dyeing has been increased, due to the variety of advantages associated with it. On the other hand, there is a growing demand for natural, eco-friendly dyeing for the health sensitive application to textile garments as an alternative to harmful synthetic dyes, which poses a need for suitable effective dyeing methodologies [ 62 ].
Ultrasonic energy can clean or homogenize materials, accelerating both physical and chemical reactions, and these qualities can be used to improve textile processing methods. Environmental concern has been focused on textile processing methods for quite some time, and the use of ultrasonic energy has been widely studied in terms of improving washing fastness. The textile dyeing industry has long been struggling to cope with high energy costs, rapid technological changes and the need for a faster delivery time, and the effective management of ultrasonic energy could reduce energy costs and improve productivity [ 35 ]. Ultrasonic waves are vibrations with frequencies above 17 kHz, out of the audible range for humans, requiring a medium with elastic properties for propagation. The formation and collapse of the bubbles formed by ultrasonic waves (known as cavitation) is generally considered to be responsible for most of the physical and chemical effects of ultrasound in solid/liquid or liquid/liquid systems [ 63 ]. Cavitation is the formation of gas-filled microbubbles or cavities in a liquid, their growth, and under proper conditions, their implosive collapse [ 59 ].
It has been reported that ultrasonic energy can be applied successfully to wet textile processes, for example laundering, desizing, scouring, bleaching, mercerization of cotton fabrics, enzymatic treatment, dyeing and leather processing, together with the decoloration/mineralization of textile dyes in waste water [ 60 ].
In addition, ultrasonic irradiation shows promise, and has the potential, for use in environmental remediation, due to the formation of highly concentrated oxidizing species such as hydroxyl radicals (HO•), hydrogen radicals (H•), hydroperoxyl radicals (HO 2 • ) and H 2 O 2 , and localized high temperatures and pressures [ 59 ]. Therefore, the use of ultrasonic energy could indeed reduce the environmental impact caused by the textile industry.
3. Finishing and waste water
The contamination of natural waters has become one of the biggest problems in modern society, and the economical use of this natural resource in production processes has gained special attention, since in predictions for the coming years, the amount of water required per capita is of concern. This environmental problem is related not only to its waste through misuse, but also to the release of industrial and domestic effluents [ 64 ].
Of the industries with high-polluting power, the textile dyeing industry, responsible for dyeing various types of fiber, stands out. Independent of the characteristics of the dyes chosen, the final operation of all dyeing process involves washing in baths to remove excesses of the original or hydrolyzed dyes not fixed to the fiber in the previous steps [ 36 ]. In these baths, as previously mentioned, it is estimated that approximately 10-50% of the dyes used in the dyeing process are lost, and end up in the effluent [ 17 , 21 , 22 ], contaminating the environment with about one million tons of these compounds [ 65 ]. The dyes end up in the water bodies due mainly to the use of the activated sludge treatment in the effluent treatment plants, which has been shown to be ineffective in removing the toxicity and coloring of some types of dye [ 33 , 60 , 66 , 67 ]. Moreover, the reduction of azo dyes by sodium hydrosulfite and the successive chlorination steps with hypochlorous acid, can form 2-benzotriazoles fenilbenzotriazol (PBTA) derivatives and highly mutagenic aromatic amines, often more mutagenic than the original dye [ 68 ]. In an aquatic environment, this dye reduction can occur in two phases: 1) The application of reducing agents to the newly-dyed fibers to remove the excess unbound dye, which could lead to "bleeding" of the fabrics during washing, and 2) The use of reducing agents in the bleaching process, in order to make the effluent colorless and conform with the legislation. This reduced colorless effluent containing dyes is sent to the municipal sewage treatment plant, where they chlorinate the effluents before releasing them into water bodies where they may generate PBTAs. Several different PBTAs are already described in the literature, and their chemical structures vary depending on the dyes that originated them [ 63 , 69 ].
So the release of improperly treated textile effluents into the environment can become an important source of problems for human and environmental health. The major source of dye loss corresponds to the incomplete fixation of the dyes during the textile fiber dyeing step [ 36 ].
In addition to the problem caused by the loss of dye during the dyeing process, within the context of environmental pollution, the textile industry is also focused due to the large volumes of water used by its industrial park, consequently generating large volumes of effluent [ 64 ]. It has been calculated that approximately 200 liters of water are needed for each kilogram of cotton produced [ 70 ]. These effluents are complex mixtures of many pollutants, ranging from original colors lost during the dyeing process, to associated pesticides and heavy metals [ 71 ], and when not properly treated, can cause serious contamination of the water sources [ 64 ]. So the materials that end up in the water bodies are effluents containing a high organic load and biochemical oxygen demand, low dissolved oxygen concentrations, strong color and low biodegradability. In addition to visual pollution, the pollution of water bodies with these compounds causes changes in the biological cycles of the aquatic biota, particularly affecting the photosynthesis and oxygenation processes of the water body, for example by hindering the passage of sunlight through the water [ 72 ].
Moreover, studies have shown that some classes of dye, especially azo dyes and their by-products, may be carcinogenic and / or mutagenic [ 27 , 33 , 67 , 73 - 77 ], endangering human health, since the wastewater treatment systems and water treatment plants (WTP) are ineffective in removing the color and the mutagenic properties of some dyes [ 78 , 79 ]. The difficulty in removing them from the environment can be attributed to the high stability of these compounds, since they are designed to resist biodegradation to meet the demands of the consumer market with respect to durability of the colors in the fibers, consequently implying that they also remain in the environment for a long time [ 32 ].
With respect to the legislation, there is no consensus amongst the different countries concerning effluent discharge, and there is no official document listing the different effluent limit values applied in different countries. Many federal countries, such as the United States of America, Canada and Australia have national environmental legislation, which, as in Europe, establishes the limits that must be complied with. Some countries, such as Thailand, have copied the American system, whereas others, such as Turkey or Morocco, have copied the European model. In some countries, for example India, Pakistan and Malaysia, the emission limits are recommended, but are not mandatory [ 80 ]. With respect to the color, in some countries such as France, Austria and Italy, there are limits for the color of the effluent, but since they use different units, a comparison is impossible. The oldest unit is the Hazen, in use since the beginning of the 20th century, but in France, the current unit is (mg L -1 Pt–Co). The coloration values are determined by a comparative analysis with model solutions prepared according to defined procedures [ 80 ].
Based on all the problems cited above regarding the discharge of effluents into the environment, it is obvious there is a need to find alternative treatments that are effective in removing dyes from effluents.
4. Waste water treatment
These days, environmental pollution can undoubtedly be regarded as one of the main problems in developed and developing countries. This is due, not just to one, but to a number of factors, such as the misuse of natural resources, inefficient legislation and a lack of environmental awareness. Fortunately, in recent years there has been a trend for change and a series of scientific studies are being used as an important tool in the development of new treatment technologies and even in the implementation of processes and environmentally friendly actions [ 64 , 81 - 84 ].
Every industrial process is characterized by the use of inputs (raw materials, water, energy, etc.) that undergo transformation giving rise to products, byproducts and waste. The wastes produced at all stages of the various types of human activity, both in terms of composition and volume, vary according to the consumption practices and production methods. The main concerns are focused on the impact these can have on human health and the environment. Hazardous waste, produced mainly by industry, is particularly worrying, because when incorrectly managed, it becomes a serious threat to the environment and therefore to human health. Thus the study of new alternatives for the treatment of different types of industrial effluent continues to be a challenge to combat anthropogenic contamination.
Amongst that of several other industries, the textile sector waste has received considerable attention in recent years, since it can generate large volumes of effluents that, if not correctly treated before being disposed into water resources, can be a problem, as previously mentioned. Effluents from the textile industry are extremely complex, since they contain a large variety of dyes, additives and derivatives that change seasonally, increasing the challenge to find effective, feasible treatments. Currently, the processes developed and available for these industries are based on methods that were designed for other waste, and have limitations when applied to textile effluents. As a consequence, these industries produce colored wastewater with a high organic load, which can contribute enormously to the environmental pollution of surface water and treatment plants if not properly treated before disposal into the water resources [ 85 ]. The ingestion of water contaminated with textile dyes can cause serious damage to the health of humans and of other living organisms, due to the toxicity, highlighting mutagenicity of its components [ 86 , 87 ]. Therefore treatments that are more efficient and economical than those currently available are required.
There are several techniques for the treatment of effluents, such as incineration, biological treatment, absorption onto solid matrices, etc. However, these techniques have their drawbacks, such as the formation of dioxins and furans, caused by incomplete combustion during incineration; long periods for biological treatment to have an effect, as also the adsorptive process, that is based on the phase transfer of contaminants without actually destroying them [ 88 , 89 ]. The problem is further aggravated in the textile industry effluents, due to the complexity of their make-up. Thus it can be seen that processes are being used that are not entirely appropriate for the treatment of textile effluents, thereby creating a major challenge for the industry and laundries that need to adapt to current regulations for the control of the color of effluents with a high organic load.
The use of filtration membranes and/or separation [ 90 ] and biological methods [ 91 ], in addition to incineration processes involving adsorption onto solid matrices, has also being adopted by the textile industry and is receiving considerable attention. However, all these processes only involve phase transfer, generating large amounts of sludge deposited at the end of the tanks and low efficiency in color removal and reduction of the organic load. According to this scenario, many studies have been carried out with the aim of developing new technologies capable of minimizing the volume and toxicity of industrial effluents. Unfortunately, the applicability of these types of system is subject to the development of modified procedures and the establishment of effluent recycling systems, activities that imply evolutionary technologies and which are not yet universally available. Thus the study of new alternatives for the treatment of many industrial effluents currently produced is still one of the main weapons to combat the phenomenon of anthropogenic contamination.
Due to their considerable danger, several authors have attempted to find new forms of treatment to reduce the serious environmental and toxicological risks caused by various organic compounds. Amongst the many reported cases are those based on the use of specific microorganisms, and degradation using advanced oxidation processes (AOP) such as Fenton, photo-Fenton and heterogeneous photocatalysis, which are highlighted below.
The use of microorganisms cultivated specifically for the degradation of polluents to increase the yield of degradation, has been reported by some authors. For example, Flores et al. (1997) examined the behavior of 25 N-substituted aromatic compounds such as organic compounds,azo dyes and nitro, using the methanogenic bacteria acetoclastic, and found that under anaerobic conditions it was easy to mineralize various of the compounds evaluated with a good yield, especially the nitroaromatic and azo dyes [ 91 ].
Bornick et al. (2001) evaluated the use of aerobic microorganisms to degrade aromatic amines present in sediments of the river Elbe in Germany. The results obtained showed that it was possible to predict the qualitative degradation of the aromatic amines using degradation constants [ 92 ].
Using a structural design, Wang et al.(2007) isolated a bacterium capable of promoting the degradation of the compounds pentyl amine and aniline present in water oil extraction in China. Under conditions of neutral pH and complete aeration of 6 mg O 2 / l at a temperature of 30 ° C, they obtained degradation yields of 82% and 78%, respectively, for pentyl amine and aniline [ 93 ].
However, in general, although the use of microorganisms in the treatment of industrial and laboratory wastes containing aromatic amines deserves attention, mainly due to the low investment and maintenance costs, the results are far from ideal, due to the low biodegradation yields, long treatment times, and the generation of sludge deposited at the bottom of the treatment ponds [ 94 ].
Of the studies carried out using a source of hydroxyl radicals with oxidizing agents, the photo Fenton system developed by Fukushima et al., 2000 to promote the degradation of aniline stands out. This method has shown promise for the mineralization of aromatic amines, obtaining a reduction of approximately 85%. However, high performance liquid chromatography (HPLC) identified a number of intermediate species formed during the degradation of the aniline, such as p-aminophenol, p-hydroquinone, maleic and fumaric acids and NH 4 + [ 95 ].
Studies involving heterogeneous photocatalysis also deserve attention: for example Pramauro et al., (1995) promoted the degradation of various aniline derivatives using TiO 2 particles suspended in a solution. Under optimal conditions, the method developed showed rapid mineralization of the aromatic amines examined in less than 1 hour of analysis. By-products generated during the degradation of these compounds were identified at the start of the reaction, but none were identified at the end of the reaction. The use of solar radiation was also evaluated, but was shown to be less efficient than artificial radiation [ 96 ].
Augugliaro et al.(2000), confirmed that heterogeneous photocatalysis using TiO 2 as a semiconductor may be a suitable method for the complete photodegradation of aniline, 4-ethylaniline and 4-chloroaniline in an aqueous medium. The kinetic parameters for the Langmuir-Hinshelwood model were used to describe the importance of the adsorption results, which proved to be independent of the pH of the solution and of the type of substituent on the aromatic ring of the amine [ 97 ].
Canle et al. (2005) studied the behavior of the adsorption of aniline and dimethyl aniline onto three species of TiO 2 (P25, anatase and rutile) and established that the greatest adsorption occurred onto TiO 2 P25. The effect of pH on the degradation provided by these compounds was also evaluated, and lower mineralization percentages were observed in acidic media. This type of behavior can be attributed to the positive charges on both the aniline and the semiconductor, providing electrostatic repulsion between the two species. Thus, an alkaline medium has been recommended as the most appropriate one to promote the mineralization of aromatic amines using a TiO 2 P25 type semiconductor [ 98 ].
Chu et al. (2007) observed the effects of pH variation and the addition of hydrogen peroxide on the degradation of 2-chloroaniline using TiO 2 as a semiconductor, with and without the application of UV radiation. The results showed that the addition of low concentrations of H 2 O 2 to the UV/TiO 2 system provided a significant increase in degradation of the aromatic amine. The addition of an excess of H 2 O 2 promoted no increase in degradation, as expected; to the contrary, a reduction in the reaction rate was observed. The variation in pH was evaluated in both systems, and the condition leading to the highest percentages of mineralization was obtained in an alkaline medium using H 2 O 2 /UV/TiO 2 [ 99 ].
Low et al. (1991) monitored the inorganic products resulting from the degradation of several organic nitrogenated, sulfured and halogenated compounds. Degradation was carried out using TiO 2 as the semiconductor and artificially illuminated UV radiation. Ammonium ions were found to be present in higher concentrations than nitrate ions, which can be explained by the fact that compounds having a nitrogen element in their structure pass through a complex degradation step where the generation of ammonium ions is more favorable than the generation of nitrate ions. In turn, compounds with nitro groups in their structures, showed higher concentrations of nitrate ions. Under ideal conditions, all the elements were converted into their respective inorganic forms. Organic carbon was converted to CO 2 , the halogens to their corresponding halide, sulfur compounds to sulfate, the phosphate to phosphorus and nitrogen to ammonium and nitrate [ 100 ]. However, all these studies used a photocatalytic titanium suspension, requiring a subsequent step to remove the semiconductor.
With a view to these problems, a new technique that has been studied recently, with significant success, is the oxidation of organic matter via the generation of hydroxyl radicals (OH•). This kind of effluent treatment has been highlighted for its destructive character with respect to the organic matter. The advanced oxidation processes (AOP) are processes based on the generation of highly oxidizing species, the hydroxyl radical (OH•), which can oxidize the organic contaminants present in water, air or soil. These radicals have a high oxidation potential (E * = +2.72 V vs. the normal hydrogen electrode, NHE), which results in high reactivity with organic pollutants. This may initiate different types of reaction with different functional groups in organic compounds, forming unstable organic radicals which are then easily oxidized to CO 2 , H 2 O and inorganic acids, derived from this heteroatom. Many techniques have being developed using this principle as the method of treatment, and have shown potential success in organic residue mineralization processes [ 92 , 96 , 101 - 103 ].
The association of the electrochemical properties with the photocatalytic properties of a semiconductor, have allowed for the development of a promising system, representing a very efficient technique as an AOP method that has received much attention in recent years, assigned as a photoelectrochemical process. The process gained notoriety for the possibility of forming hydroxyl radicals via the oxidation of water. The technique is based on the action of ultraviolet light (hυ) on a semiconductor capable of generating charges and e - / H + , whose separation is facilitated by applying a positive potential (E APP ), greater than the potential of a flat band photocatalytic material. The generation of a potential gradient in the photoactivated semiconductor directs the electrons to an auxiliary electrode (cathode), delaying recombination between the holes (h + ) generated in the valence band (VB), and making them available for oxidation processes and the generation of hydroxyl radicals of interest [ 104 ].
The results of this process were very promising because of the relatively short treatment time but with great efficiency, both in the removal of color and in the reduction of the organic load. However, the limitations of this technique are related mainly to the choice of the ideal catalyst for promoting the generation of these oxidizing species. Catalysts that promote the generation of radicals absorbing radiation in the visible spectral region are the most desirable for this type of reaction, due to the large percentage emitted in the solar spectrum (approximately 45%) [ 104 ].
Thus, the development of an ideal process that promotes color removal and a reduction in the organic load of wastewater from the textile industry with great efficiency is a major challenge in all fields of science, since the synthesis of the best catalyst to take advantage of solar radiation, thus reducing the operating costs, and at the same time solve the problems involved in the hydrodynamics of the reactors, is of importance in the development of the treatment.
The expectations for developing an effective method for the treatment of these wastes are quite promising, but require continuous optimization and knowledge of new aspects. These include better fixation of the dyes to the fibers, process with less water consumption, less hazardous dyes with respect to human health and methods capable of identifying these compounds with more efficacy and rapidity and assays to identify any potential carcinogenic and / or mutagenic properties in the dyes and their derivatives; genetic improvements to produce more efficient culture mediums and resistant biological treatments, leading to a reduction in the generation of sludge; the synthesis of materials that catalyze reactions in the visible spectral regions, leading to a more economic photoeletrochemical method, and also new engineering advances for the construction of more effective reactors, which can take advantage of all these developments in an integrated system, extending the performance of a process more appropriate for the treatment of such a complex effluent.
5. Optimization of the dyeing processes to reduce the environmental impact of the textile industry
The search and development of new methods to promote the treatment of effluents from the textile industry with a maximum of efficiency of the process of decolorization and / or removal of these compounds present in the medium can trigger further damage human health and the environment is fundamental importance. The understanding of the composition of waste generated is extremely significant to develop these methods of treatment due to the high complexity by virtue of huge number of compounds which are added at different stages of the dyeing fabrics.
Environmental problems with used dye baths are related to the wide variety of different components added to the dye bath, often in relatively high concentrations. In the future, many of textile factories will face the requirement of reusing a significant part of all incoming freshwater because traditionally used methods are insufficient for obtaining the required water quality.
However, due to dwindling supply and increasing demand of water in the textile industries, a better alternative is to attempt to further elevate the water quality of wastewater effluent from a secondary wastewater treatment plant to a higher standard for reuse. Thus far very little attention has been paid to this aspect [ 105 ].
Therefore, the investment in the search for methodologies to more effective treatment of these effluents can be much smaller than that spent in tertiary treatment to remove these products in low level of concentrations and in the presence of much other interference. This requires action that the cost / benefit are reviewed and the development of new techniques for wastewater treatment capable of effective removal of these dyes is intensified and made economically viable [ 105 , 106 ].
An alternative to minimize the problems related to the treatment of textile effluents would be the development of more effective dye that can be fixed fiber with higher efficiency decreasing losses on tailings waters and reducing the amount of dye required in the dyeing process, reducing certainly improve the cost and quality of the effluent.
6. Conclusion
It was concluded that the synthetic textile dyes represent a large group of organic compounds that could have undesirable effects on the environment, and in addition, some of them can pose risks to humans. The increasing complexity and difficulty in treating textile wastes has led to a constant search for new methods that are effective and economically viable. However, up to the present moment, no efficient method capable of removing both the color and the toxic properties of the dyes released into the environment has been found.
Acknowledgments
This work was supported by the Faculty of Pharmaceutical Sciences at Ribeirão Preto - University of São Paulo, Brazil, and by FAPESP, CAPES and CNPq.
- 1. Kirk-Othmer. Encyclopedia of Chemical Technology, v. 7, 5th Edition. Wiley-Interscience; 2004.
- 2. Bafana A, Devi SS, Chakrabarti T. Azo dyes: past, present and the future. Environmental Reviews 2011; 19 350–370.
- 3. Zollinger, H. Synthesis, Properties of Organic Dyes and Pigments. In: Color Chemistry. New York, USA: VCH Publishers; 1987. p. 92-102.
- 4. Carneiro PA, Nogueira, RFP, Zanoni, MVB. Homogeneous photodegradation of C.I. Reactive Blue 4 using a photo-Fenton process under artificial and solar irradiation. Dyes and Pigments 2007; 74 127-132.
- 5. Christie R. Colour Chemistry. Cambridge, United Kingdom: The Royal Society of Chemistry; 2001.
- 6. Dos Santos AB, Cervantes FJ, van Lier JB. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresource Technology 2007; 98 (12) 2369-2385.
- 7. Arun Prasad AS, Bhaskara Rao KV. Physico chemical characterization of textile effluent and screening for dye decolorizing bactéria. Global Journal of Biotechnology and Biochemistry 2010; 5(2) 80-86.
- 8. Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technology 2001; 77 (12) 247-255.
- 9. Ogugbue CJ, Sawidis T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Biotechnology Research International 2011; DOI 10.4061/2011/967925.
- 10. Perkins WS. A Review of Textile Dyeing Processes. American Association of Textile Chemists and Colorists 1991; 23 (8): 23–27.http://www.revistavirtualpro.com/files/TIE08_200704.pdf (accessed 12 May 2012)
- 11. Couto SR. Dye removal by immobilised fungi. Biotechnology Advances 2009; 27(3) 227-235.
- 12. O’Neill C, Hawkes FR, Hawkes DL, Lourenço ND, Pinheiro HM, Delée W. Colour in textile effluents – sources, measurement, discharge consents and simulation: a review. Journal of Chemical Technology and Biotechnology 1999; 74 (11) 1009-1018.
- 13. Forgacs E, Cserháti T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environment International 2004; 30 (7) 953- 971
- 14. Przystaś W, Zabłocka-Godlewska E, Grabińska-Sota E. Biological Removal of Azo and Triphenylmethane Dyes and Toxicity of Process By-Products. Water Air Soil Pollut 2012; 223 (4) 1581-1592.
- 15. Sen S, Demirer GN. Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Research 2003; 37 (8) 1868-1878.
- 16. Dos Santos, A B. Reductive Decolourisation of Dyes by Thermophilic Anaerobic Granular Sludge. PhD-Thesis. Wageningen University, The Netherlands, 2005.
- 17. Ben Mansour H, Houas I, Montassar F, Ghedira K, Barillier D, Mosrati R, Chekir-Ghedira L. Alteration of in vitro and acute in vivo toxicity of textile dyeing wastewater after chemical and biological remediation. Environmental science and pollution research international 2012; DOI 10.1007/s11356-012-0802-7.
- 18. Talarposhti AM, Donnelly T, Anderson GK. Colour removal from a simulated dye wastewater using a two-phase anaerobic packed bed reactor. Water Research 2001; 35 (2) 425-432.
- 19. Ibrahim MB, Poonam N, Datel S, Roger M. Microbial decolorization of textile dye-containing effluents: a review, Bioresource Technology 1996; 58(3) 217-227.
- 20. Wijetunga S, Li XF, Jian C. Effect of organic load on decolourization of textile wastewater containing acid dyes in upflow anaerobic sludge blanket reactor. Journal of Hazardous Materials 2010; 177 (1-3) 792-798.
- 21. Vaidya AA, Datye KV. Environmental pollution during chemical processing of synthetic fibers. Colourage 1982; 14 3-10.
- 22. Rajaguru P, Fairbairn LJ, Ashby J, Willington MA, Turner S, Woolford LA, Chinnasamy N, Rafferty JA. Genotoxicity studies on the azo dye Direct Red 2 using the in vivo mouse bone marrow micronucleus test. Mutation Research 1999; 444(1) 175-180.
- 23. Hubbe MA, Beck KR, O’Neal WG, Sharma YC. Cellulosic substrates for removal of pollutants from aqueous systems: a review. 2. Dyes. Dye biosorption: Review. BioResources 2012; 7 (2), 2592-2687.
- 24. Carliell CM, Barclay SJ, Shaw C, Wheatley AD, Buckley C A. The effect of salts used in textile dyeing on microbial decolourisation of a reactive azo dye. Environmental Technology 1998; 19 (11) 1133-1137.
- 25. Seesuriyachan P, Takenaka S, Kuntiya A, Klayraung S, Murakami S, Aoki K. Metabolism of azo dyes by Lactobacillus casei TISTR 1500 and effects of various factors on decolorization. Water Research 2007; 41(5) 985-992.
- 26. Ben Mansour H, Corroler D, Barillier D, Ghedira K, Chekir L, Mosrati R. Evaluation of genotoxicity and pro-oxidant effect of the azo dyes: Acids yellow 17, violet 7 and orange 52, and of their degradation products by Pseudomonas putida mt-2. Food and Chemical Toxicology 2007; 45 (9) 1670-1677.
- 27. Chung KT, Cerniglia CE. Mutagenicity of azo dyes: Structure-activity relationships. Mutation Research, 1992; 277 (3) 201-220.
- 28. Pinheiro HM, Touraud E, Thomas O. Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes and Pigments 2004; 61 (2) 121-139.
- 29. Umbuzeiro GA, Freeman H, Warren SH, Kummrow F, Claxton LD. Mutagenicity evaluation of the commercial product CI Disperse Blue 291 using different protocols of the Salmonella assay. Food and Chemical Toxicology 2005; 43 (1) 49-56.
- 30. Arlt VM, Glatt H, Muckel E, Pabel U, Sorg BL, Schmeiser HH, Phillips DH. Metabolic activation of the environmental contaminant 3-nitrobenzanthrone by human acetyltransferases and sulfotransferase. Carcinogenesis 2002; 23 (11) 1937-1945.
- 31. Hao OJ, Kim H, Chiang PC, 2000. Decolorization of wastewater. Critical Reviews in Environmental Science and Technology 2000; 30 (4) 449-505.
- 32. Firmino PIM, Silva MER, Cervantes FJ, Santos AB. Colour removal of dyes from synthetic and real textile wastewaters in one- and two-stage anaerobic systems. Bioresource Technology 2010; 101(20) 7773-7779.
- 33. Carneiro PA, Umbuzeiro GA, Oliveira DP, Zanoni MVB. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. Journal of Hazardous Materials 2010; 174 (1-3) 694-699.
- 34. Barani H, Montazer M. A Review on Applications of Liposomes in Textile Processing. Journal of Liposome Research 2008; 18 (3) 249-262.
- 35. Khatri Z, Memon MH, Khatri A, Tanwari A. Cold Pad-Batch dyeing method for cotton fabric dyeing with reactive dyes using ultrasonic energy. Ultrasonics Sonochemistry 2011 18 (6) 1301-1307.
- 36. Guaratini CCI, Zanoni MVB. Textile dyes. Química Nova 2000;23(1): 71-78. http://www.scielo.br/pdf/qn/v23n1/2146.pdf (accessed 01 May 2012).
- 37. Moore SB, Ausley LW. Systems thinking and green chemistry in the textile industry: concepts, technologies and benefits. Journal of Cleaner Production 2004;12 585–601. DOI: 10.1016/S0959-6526(03)00058-1
- 38. Reddy SS, Kotaiah B, Reddy NSP. Color pollution control in textile dyeing industry effluents using tannery sludge derived activated carbon. Bulletin of the Chemical Society of Ethiopia 2008;22 (3): 369-378. http://www.ajol.info/index.php/bcse/article/viewFile/61211/49389 (accessed 16 May 2012).
- 39. Alcantara MR, Daltin D. A química do processamento têxtil. Química Nova 1996;19(3): 320-330 http://www.quimicanova.sbq.org.br/qn/qnol/1996/vol19n3/v19_n3_17.pdf (accessed 19 April 2012).
- 40. Vassileva V, Valcheva E, Zheleva Z. The kinetic model of reactive dye fixation on cotton fibers. Journal of the University of Chemical Technology and Metallurgy 2008;43 (3): 323-326. http://www.uctm.edu/j2008-3/8_V_Vasileva_323-326.pdf (accessed 10 May 2012).
- 41. Zollinger H. Color Chemistry. Syntheses,properties and applications of organic dyes and pigments. Weinheim: VCH Publishers; 2003.
- 42. Candlin J. Polymers. Dyestuffs in the Chemical Industry. London: Chapman & Hall; 1994.
- 43. Gregory P. Dyestuffs. In: Heaton C.A. (ed.) The Chemical Industry. London: Chapman & Hall; 1994. p143-188.
- 44. Gregory P. 2009. Dyes and Dye Intermediates. In: Kirk-Othmer (ed.) Encyclopedia of Chemical Technology. New Jersey: John Wiley & Sons; 2009. p1–66. DOI: 10.1002/0471238961.0425051907180507.a01.pub2
- 45. Pang K, Kotek R, Tonelli A. Review of conventional and novel polymerization processes for polyesters. Progress in Polymer Science 2006;31(11) 1009–1037. DOI: 10.1016/j.progpolymsci.2006.08.008
- 46. Hunger K., editor. Industrial Dyes: Chemistry, Properties, Applications. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2004. DOI: 10.1002/3527602011
- 47. Baptista ALF, Coutinho PJG,: Real Oliveira MECD, Rocha Gomes JIN. Effect of surfactants in soybean Lecithin liposomes studied by energy transfer between NBD-PE and N-Rh-PE. Journal of Liposome Research 2000; 10 (4) 419-429.
- 48. Lesoin L, Crampon C, Boutin O, Badens E. Preparation of liposomes using the supercritical anti-solvent (SAS) process and comparison with a conventional method. The Journal of Supercritical Fluids 2011; 57 162-174
- 49. Sivasankar, M, Katyayani, T. Liposomes – The future of formulations. International Journal of Research in Pharmacy and Chemistry 2011; 1(2) 259-267.
- 50. El-Zawahry MM, El-Shami S, El-Mallah S.H. Optimizing a wool dyeing process with reactive dye by liposome microencapsulation. Dyes and Pigments 2007; 74 684-691.
- 51. Martí M, de la Maza A, Parra JL, Coderch L. Liposome as dispersing agent into disperse dye formulation. Textile Research Journal 2011; 81(4) 379-387.
- 52. Montazer M., Validi M., Toliyat T. Influence of Temperature on Stability of Multilamellar Liposomes in Wool Dyeing. Journal of Liposome Research, 2006; 16:81-89.
- 53. Martí M, Coderch L, de la Maza A, Parra JL. Liposomes of phosphatidylcholine: a biological natural surfactant as a dispersing agent. Color Technology 2007; 123 237-241.
- 54. Riaz M. Liposomes preparation methods. Pakistan Journal of Pharmaceutical Sciences, 1996; 19(1) 65-77.
- 55. Martí M, de la Maza A, Parra JL, Coderch L. Liposome as dispersing agent into disperse dye formulation. Textile Research Journal 2010; 81(4) 379–387.
- 56. Nelson, G. Application of microencapsulation in textile. International Journal of Pharmaceutics 2002; 242 55-62.
- 57. Martí M, de la Maza A, Parra JL, Coderch L. Dyeing wool at low temperatures: new method using liposomes. Textile Research Journal 2001; 71(8) 678-682.
- 58. El-Zawahry MM, El-Mallahb MH, El-Shamib S. An innovative study on dyeing silk fabrics by modified phospholipid liposomes. Coloration Technology 2009; 125 164-171.
- 59. Vajnhandl S, Le Marechal AM. Ultrasound in textile dyeing and the decolouration/mineralization of textile dyes. Dyes and Pigments 2005; 65 89-101.
- 60. Ferrero F., Periolatto M. Ultrasound for low temperature dyeing of wool with acid dye. Ultrasonics Sonochemistry 2012; 19 601-606.
- 61. Vankar PS, Shanker R. Ecofriendly ultrasonic natural dyeing of cotton fabric with enzyme pretreatments. Desalination 2008; 230 62-69.
- 62. Mansour HF, Heffernan S. Environmental aspects on dyeing silk fabric with sticta coronate lichen using ultrasonic energy and mild mordants. Clean Technologies Environmental Policy 2011; 13 207-213.
- 63. Abou-Okeil A, El-Shafie A, El Zawahry MM. Ecofriendly laccase–hydrogen peroxide/ultrasound-assisted bleaching of linen fabrics and its influence on dyeing efficiency. Ultrasonics Sonochemistry 2010; 17 383-390.
- 64. Kunz A, Zamora PP, Moraes SG, Durán N. New tendencies on the textile effluent treatment. Quimica Nova 2002;25(1): 78-82. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-40422002000100014 (accessed 01 May 2012).
- 65. Associação Brasileira de Química. ABQ. Processos oxidativos avançados e tratamento de corantes sintéticos. Anais da ABQ. http://www.unb.br/resqui/abq2004-1.pdf (accessed 18 May 2012).
- 66. Umbuzeiro GA, Roubicek DA, Rech CM, Sato MIZ, CLAXTON LD. Investigating the sources of the mutagenic activity found in a river using the Salmonella assay and different water extraction procedures. Chemosphere 2004; 54(11) 1589-1597. DOI: 10.1016/j.chemosphere.2003.09.009
- 67. Umbuzeiro GA, Freeman HS, Warren SH, Oliveira DP, Terao Y, Watanabe T, Claxton LD. The contribution of azo dyes to the mutagenic activity of the Cristais river. Chemosphere 2005; 60 (1) 55-64. DOI:10.1016/j.chemosphere.2004.11.100
- 68. Shiozawa T, Suyama K, Nakano K, Nukaya H, Sawanishi H, Oguri A, Wakabayashi K, Terao Y. Mutagenic activity of 2 phenylbenzotriazole derivatives related to a mutagen, PBTA-1, in river water. Mutation Research 1999; 442 (2) 105-111.
- 69. Oliveira DP. Corantes como importante classe de contaminantes ambientais – um estudo de caso. PhD thesis. Universidade de São Paulo; 2005.
- 70. Carneiro PA, Osugi ME, Sene JJ, Anderson MA, Zanoni MVB. Evaluation of color removal and degradation of a reactive textile azo dye on nanoporous TiO thin-film electrodes. Electrochimica Acta 2004; 49 (22-23) 3807–3820. DOI: 10.1016/j.electacta.2003.12.057
- 71. McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat IM, Marchant R, Smyth WF. Mini-review: microbial decolorisation and degradation of textile dyes. Applied Microbiology and Biotechnology 2001; 56 (1-2): 81-87. DOI: 10.1007/s002530000587. http://www.springerlink.com/content/04vuqljhajpa32rv/ (accessed 10 April 2012).
- 72. Pereira WS, Freire RS. Ferro zero: Uma nova abordagem para o tratamento de águas contaminadas com compostos orgânicos poluentes. Química Nova 2005;28(1): 130-136. DOI: 10.1590/S0100-40422005000100022. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-40422005000100022 (accessed 10 March 2012).
- 73. Houk VS. The genotoxicity of industrial wastes and effluents. Mutation Research 1992; 277 (2) 91-138.
- 74. Jager I, Hafner C, Schneider K. Mutagenicity of different textile dye products in Salmonella typhimurium and mouse lymphoma cells. Mutation Research 2004;561 (1-2) 35-44.
- 75. Chequer FMD, Angeli JPF, Ferraz ERA, Tsuboy MS, Marcarini JC, Mantovani MS, Oliveira DP. The azo dyes Disperse Red 1 and Disperse Orange 1 increase the micronuclei frequencies in human lymphocytes and in HepG2 cells. Mutation Research 2009; 676 (1-2) 83-86.
- 76. Chequer FMD, Lizier TM, Felicio R, Zanoni MVB, Debonsi HM, Lopes NP, Marcos R, Oliveira DP. Analyses of the genotoxic and mutagenic potential of the products formed after the biotransformation of the azo dye Disperse Red 1. Toxicology in Vitro 2011; 25 (8) 2054-2063
- 77. Ferraz ERA, Umbuzeiro GA, de-Almeida G, Caloto-Oliveira A, Chequer FMD, Zanoni MVB, Dorta DJ, Oliveira DP. Differential Toxicity of Disperse Red 1 and Disperse Red 13 in the Ames Test, HepG2 Cytotoxicity Assay, and Daphnia Acute Toxicity Test. Environmental Toxicology 2011; 26 (5) 489-497
- 78. Oliveira GAR, Ferraz ERA, Chequer FMD, Grando MD, Angeli JPF, Tsuboy MS, Marcarini JC, Mantovani MS, Osugi ME, Lizier TM, Zanoni, MVB, Oliveira DP. Chlorination treatment of aqueous samples reduces, but does not eliminate, the mutagenic effect of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1. Mutation Research 2010 703 (2) 200-208
- 79. Konstantinou IK, Albanis TA. TiO 2 -assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations - A review. Applied Catalysis B: Environmental 2004; 49 (1) 1-14. DOI:10.1016/j.apcatb.2003.11.010.
- 80. Hessel C, Allegre C, Maisseu M, Charbit F, Moulin P. Guidelines and legislation for dye house effluents. Journal of Environmental Management 2007; 83 (2) 171-180. DOI: 10.1016/j.jenvman.2006.02.012.
- 81. Carneiro PA, Osugi ME, Fugivara CS, Boralle N, Furlan M, Zanoni MVB. Evaluation of different electrochemical methods on the oxidation and degradation of Reactive Blue 4 in aqueous solution. Chemosphere. 2005; 59(3) 431-439.
- 82. Mohan SV, Bhaskar YV, Karthikenyan J. Biological decolourization of simulated azo dye in aqueous phase by algae Spirogyra species. International Journal of Environment and Pollution. 2004; 21 (3) 211-222.
- 83. Vaghela SS, Jethva AD, Mehta BB, Dave SP, Adimurthy S, Ramachandraiah G. Laboratory studies of electrochemical treatment of industry azo dye effluent. Environmental Science and Technology. 2005; 39 (8) 2848-2855.
- 84. Cardoso JC, Lizier TM, Zanoni MVB. Highly ordered TiO2 nanotube arrays and photoelectrocatalytic oxidation of aromatic amine. Applied Catalysis, B: Environmental. Applied Catalysis, B: Environmental. 2010; 99 (1-2) 96-102.
- 85. Pereira RS. Identificação e caracterização das fontes de poluição em sistemas hídricos. Revista Eletrônica de Recursos Hídricos. IPH-UFRGS 2004; 1(1): 20-36. http://www.abrh.org.br/informacoes/rerh.pdf (accessed 06 June 2012).
- 86. Baumgarten, MGZ.; Pozza, SA. Qualidade de Águas. Descrição de Parâmetros Químicos referidos na Legislação Ambiental. Ed: FURG; 2001.
- 87. Prado AGS, Torres JD, Faria EA, Dias SCL. Comparative adsorption studies of indigo carmine dye on chitin and chitosan. Journal of Colloid and Interface Science. 2004; 1(1) 43-47.
- 88. Babel S, Kurniawan TA. Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials. 2003; 97(1-3) 219-243.
- 89. Associação Brasileira da Indústria Têxtil e de Confecção. Economia. <http://www.abit.org.br/site/navegacao.asp?id_menu=8&IDIOMA=PT> (accessed 19 August 2011).
- 90. Walsh FC. Electrochemical technology for environmental treatment and clean energy conversion. Pure and Applied Chemistry. 2001; 73 (12) 1819-1837.
- 91. Flores ER, Perez F, Torre M. Scale-up of Bacillus thuringiensis fermentation based on oxygen transfer. Journal of Fermentation and Bioengineering. 1997; 83 (6) 561-564.
- 92. Bornick H. Simulation of biological degradation of aromatic amines in river bed sediments. Water Research. 2001; 35 (3) 619-624.
- 93. Wang L, Barrington S, Kim JW. Biodegradation of pentylamine and aniline from petrochemical wastewater. Journal of Environmental Management. 2007; 83 (2) 191-197.
- 94. Orshansky F, Narkis N. Characteristics of organics removal by pact simultaneous adsorption and biodegradation. Water Research. 1997; 31(3) 391-398.
- 95. Fukushima M, Tatsumi K, Morimoto K. The fate of aniline after a photo-fenton reaction in an aqueous system containing iron(III), humic acid, and hydrogen peroxide. Environmental Science and Technology. 2010; 34 (10) 2006-2013.
- 96. Pramauro E. et al. Photocatalytic treatment of laboratory wastes containing aromatic amines. Analyst. 1995; 120 (2) 237-242.
- 97. Augugliaro V. et al. Photodegradation kinetics of aniline, 4-ethylaniline, and 4-chloroaniline in aqueous suspension of polycrystalline titanium dioxide. Research on Chemical Intermediates. 2000; 26 (5) 413-426.
- 98. Canle LM, Santaballa JA, Vulliet E. On the mechanism of TiO2-photocatalyzed degradation of aniline derivatives. Journal of Photochemistry and Photobiology, A: Chemistry. 2005; 175 (2-3) 192-200.
- 99. Chu W, Choy WK, So TY. The effect of solution pH and peroxide in the TiO2-induced photocatalysis of chlorinated aniline. Journal of Hazardous Materials. 2007; 141 (1) 86-91.
- 100. Low, G. K. C.; McEvoy, S. R.; Matthews, R. W. Formation of nitrate and ammonium ions in titanium dioxide mediated photocatalytic degradation of organic compounds containing nitrogen atoms. Environmental Science and Technology, v. 25, n. 3, p. 460-467, 1991.
- 101. Pinheiro HM, Touraud E, Thomas O. Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes and Pigments. 2004; 61(2) 121-139.
- 102. Fukushima M, Tatsumi K, Morimoto K. The Fate of Aniline after a Photo-Fenton Reaction in an Aqueous System Containing Iron(III), Humic Acid, and Hydrogen Peroxide. Environmental Science and Technology. 2006; 34 (10) 2006-2013.
- 103. Chu W, Choy WK, So TY. The effect of solution pH and peroxide in the TiO 2 -induced photocatalysis of chlorinated aniline. Journal of Hazardous Materials. 2007; 141 (1) 86-91.
- 104. Finklea HO. Semiconductor electrodes. New York: Elsevier; 1998.
- 105. Lin SH, Chen ML. Purification of textile wastewater effluents by a combined Fenton process and the ion exchange. Desalination 1997; 109 (1) 121-130.
- 106. Mozia S. Photocatalytic membrane reactor (PMRs) in water and wastewater treatment. A review. Separation and Purification Technology 2010; 73 (2) 71-91.
© 2013 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Edited by Melih Gunay
Published: 16 January 2013
By Marija Gorjanc, Marija Gorenšek, Petar Jovančić...
7319 downloads
By Sheila Shahidi, Jakub Wiener and Mahmood Ghorannev...
7633 downloads
By Bojana Voncina and Vera Vivod
6003 downloads
IntechOpen Author/Editor? To get your discount, log in .
Discounts available on purchase of multiple copies. View rates
Local taxes (VAT) are calculated in later steps, if applicable.
Support: [email protected]

Stages of Dyeing | Theory of Dyeing | Physico-Chemical Aspect of Dyeing
Last Updated on 15/03/2023
Theory / Physico-Chemical Aspect of Dyeing Process: The physico-chemical aspect or basic theory of dyeing means to the interaction between the dye molecules and the textile fibers , which is influenced by factors such as temperature, pH, and the chemical nature of the dye and fiber. This interaction involves the formation of various bonds and forces, such as hydrogen bonding, van der Waals forces, and covalent bonding, which contribute to the stability and durability of the dye-fiber complex. Understanding the physico-chemical aspect of dyeing is important in developing effective dyeing processes and achieving desired color and performance properties in dyed textiles.

Physico chemical aspect of dyeing is one kind of system or way which shows how to a dye penetrates or enter into the fiber. It also describes how many process and time dye molecules follow to enter completely onto the fiber. In this article I will describe different stages of dyeing in textile.
Different Successive Stages of Dyeing in Textile: Dyeing is the process of adding color to fibers, yarns, fabrics, or garments. There are different stages involved in dyeing, which may vary depending on the type of fiber, dye, and equipment used. However, the stages of dyeing can be summarized as follows:
Dye dispersed in the dye bath ↓↑(Convective diffusion) Dye in the diffusion layer(boundary layer) ↓↑ (Molecular diffusion) Dye in the electrical double layer ↓↑ (Adsorption) Dye absorbed on the fiber surface ↓↑ (Diffusion) Dye diffused in the fiber ↓↑ (Fixation) Dye physically or chemically bond in the fiber
The dyeing process is essentially a distribution process. The dye is distributed over at least two phase systems, the dye bath and textile materials. When equilibrium dyeing is reached, the following subsidiary equilibrium is established.
1. Dye Dispersed in the Dye Bath: Most of the dyes in solution are in molecular and partially ionized state or exist in the form of ionic micells.
2. Dye in the Diffusion Layer: When a substrate is brought into a dye bath, a concentration gradient is created which will-make the dye molecules move or diffuse to the fiber. The dye which approaches the fiber surface must eventually diffuse through a thin liquid layer, the so-called diffusion layer, towards or onto the fiber surface.
3. Dye in the Electrical Double Layer: All textile fibers when immersed in water or aqueous solution, acquire an electrical potential after referred to as Beta potential. At the fiber surface the dye molecules must pass the electrical double layer, consists of non-solvated anions (mostly) and solvated cat ions. These positive and negative ions try to approach the fiber surface as close as possible. This layer is about 1nm thin.
4. Dye Absorbed on the Fiber Surface: The dye molecules reach the fiber surface (and the first layer of the fiber). This dye take-up at the fiber surface (absorption) occurs very rapidly and leads to a reduction of dye molecules in the immediate vicinity of this surface.
5. Dye Diffused in the Fiber: After absorption, dye diffused in the fiber. Owing to the high temperature, there is always an abundance of dye stuff molecules in the vicinity of the fiber and agitation has little effect upon the time of half dyeing.
6. Dye Physically or Chemically Bond in the Fiber: The last step of dyeing is fixation. In case of reactive dyes , the fixation is one-way. Because here dye molecules become attached to the fiber polymer by strong co-valent bonds. In case of all other dyes this fixation is two ways. Because they are fixed with fiber by weak hydrogen or salt linkage.
Factors of Dyeing: There are several factors those responsible for the anchoring of the dye molecules to the fiber. These factors include:
- pH of the dyeing solution
- Temperature of the dyeing solution
- Dye concentration
- Time of dyeing
- Presence of electrolytes
- Salt linkage/ionic bonds for protein fibre .
- Co-ordination linkage
- Hydrogen bonds
- Co-valent bonds; for cellulose fibers
- Physical forces
- Dispersion forces.
- Use of mordants or fixing agents
You may also like:
- Methods of Dyeing | Different Types of Dyeing Methods
- Classification and Characteristics of Dyes | Commercial Name of Dyes
- Typical Preparatory Process of Dyeing

Founder & Editor of Textile Learner. He is a Textile Consultant, Blogger & Entrepreneur. He is working as a textile consultant in several local and international companies. He is also a contributor of Wikipedia.
Share this Article!
Related Posts:

Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

IMAGES
VIDEO
COMMENTS
The majority of acid dyes are sodium salts of organic sulfuric acids. The substance is usually a sulfonic acid salt (D-SO 3 Na) composed of an aromatic framework with chromogen and solubilizing properties.; Acidic (anionic) dyes are water-soluble colors that are commonly used for dyeing various fabrics such as wool, silk, nylon, modified rayon, certain modified acrylics, and polyester.
A dye is a coloring material that is used for imparting color to different substances or altering the color of something. Dyes have chromophores and auxochromes which are responsible for their color and substantivity. Types of Dyes. Dyes can be classified based on various parameters. However, we'll take a look at four most pronounces ones -
7.0 Tutor -Marked Assignment 8.0 References/Further Reading 1.0 INTRODUCTION A dye can generally be described as a coloured substance that has an affinity to the substrate to which it is being applied. At the very basic level the use of colour in identifying individual components of tissue ... Type 4: Azo dyes, based on -N=N- azo structure
Structure and Properties of Dyes and Pigments Ashok Kumar, Utkarsh Dixit, Kaman Singh, Satya Prakash Gupta and Mirza S. Jamal Beg ... Based on different type of electron present in a molecule, different types of electronic transitions are possible. 3. Nomenclature The chemical names of dyes are very complicated hence trade names are more
A dye contains two groups that are chromophore and auxochrome groups in which a chromophore is a color-bearing group that is responsible for dye color whereas auxochrome is a color helper which is responsible for dye fiber reaction. Examples of dyes: Indigo dye, Phthalein dyes, Alizarin, etc. 10 Types of Dyes 1. Reactive Dyes
Basic dyes are also used in the coloring of papers. Basic dyestuffs for paper widely used in textile industries. Basic dye offered by us are used mainly in the applications of acrylic fibers such as types 42 and 75 orlon and type 61 creslan. The dye is generally used to produce bright and deep shades with superior light and wash fastness.
This study highlights the seven types of dyes, namely, azo dyes (Benkhaya et al. 2020;Ogugbue and Sawidis 2011), acid dyes (Kumar et al. 2021; Said et al. 2020), basic dyes (dos Santos et al. 2007 ...
Colour is one of the elements of nature that makes human life more aesthetic and fascinating in the world. Plants, animals, and minerals have been used as primary sources for colourants, dyes or pigments since ancient times. In our daily life, we know about many substances which have specific colours. These are the substances which are used as colourants i.e.; colour imparting species. Both ...
Nevertheless, studies of these dyes in the 1800s provided a base for development of synthetic dyes, which dominated the market by 1900. Two natural dyes, alizarin and indigo, have major significance. Alizarin is a red dye extracted from the roots of the madder plant, Rubia tinctorium. Two other red dyes were obtained from scale insects.
Leather can be dyed using a variety of dyes, including acid dyes and oil-based dyes. However, the type of dye used will depend on the type of leather and the desired outcome. Dyeing Cellulosic Fibers. Cellulosic fibers, such as viscose and jute, can be dyed using various dye types, including vat and reactive dyes. However, it is essential to ...
Dyes may be classified according to their chemical structure or by the method by which they are applied to the substrate. The dye manufacturers and dye chemists prefer the former approach of classifying dyes according to chemical type. The dye users, however, prefer the latter approach to of classification according to application method.
William Henry Perkin discovered the first organic dye, mauve or aniline, in 1856. Thousands of dyes have been discovered since then. Synthetic dyes cost less, offer a vast range of colors, and impart better properties on dyeing. These dyes soon replaced the traditional natural dyes. This handbook discusses different types of dyes and their uses.
The evolution of dyes is a testament to the ingenuity of mankind in harnessing natural resources. The earliest civilizations employed botanical, mineral, and even animal-derived sources to imbue fabrics with color, simultaneously addressing utilitarian needs and embodying cultural symbolism [2].As societies grew and became interconnected through trade and exploration, the demand for a broader ...
Dyes are of two types, i.e. synthetic and natural. Synthetic dyes are based on petroleum compound, whereas natural dyes are obtained from plant, animal, and mineral matters (Singh and Bharati 2014). Colorants are normally understood to include both pigments and dyestuff. Pigments refer mainly to inorganic salts and oxides, such as iron and ...
Both dyes have their own pros and cons and different areas of application in various industries. In addition, there are various types of dyes, including: 1. Direct Dyes. Direct dyes are those dyes applied directly to the material without requiring a mordant. They are widely used to color cellulosic fibers, such as rayon, cotton, and viscose. 2.
(a) Natural dyes: Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources—roots, berries, bark, leaves, and wood—and other biological sources such as fungi and lichens. There are two types of natural dyes.
2. Cost-Effective: Basic dyes are often more cost-effective than some other types of dyes, making them a preferred choice for industries that require large-scale coloration. 3. Long-Lasting Colors: Basic dyes are known for their excellent colorfastness, ensuring that the colors remain vibrant and stable over time. This is particularly important ...
1. Introduction. Dyes may be defined as substances that, when applied to a substrate provide color by a process that alters, at least temporarily, any crystal structure of the colored substances [1,2].Such substances with considerable coloring capacity are widely employed in the textile, pharmaceutical, food, cosmetics, plastics, photographic and paper industries [3,4].
Last Updated on 15/03/2023 . Theory / Physico-Chemical Aspect of Dyeing Process: The physico-chemical aspect or basic theory of dyeing means to the interaction between the dye molecules and the textile fibers, which is influenced by factors such as temperature, pH, and the chemical nature of the dye and fiber.This interaction involves the formation of various bonds and forces, such as hydrogen ...