Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 17 July 2024

Exploring treatment options in cancer: tumor treatment strategies
- Beilei Liu ORCID: orcid.org/0000-0002-0623-9093 1 , 2 , 3 na1 ,
- Hongyu Zhou 2 na1 ,
- Licheng Tan 2 na1 ,
- Kin To Hugo Siu 2 &
- Xin-Yuan Guan ORCID: orcid.org/0000-0002-4485-6017 1 , 2 , 3 , 4 , 5
Signal Transduction and Targeted Therapy volume 9 , Article number: 175 ( 2024 ) Cite this article
15k Accesses
7 Citations
8 Altmetric
Metrics details
- Drug development
Traditional therapeutic approaches such as chemotherapy and radiation therapy have burdened cancer patients with onerous physical and psychological challenges. Encouragingly, the landscape of tumor treatment has undergone a comprehensive and remarkable transformation. Emerging as fervently pursued modalities are small molecule targeted agents, antibody-drug conjugates (ADCs), cell-based therapies, and gene therapy. These cutting-edge treatment modalities not only afford personalized and precise tumor targeting, but also provide patients with enhanced therapeutic comfort and the potential to impede disease progression. Nonetheless, it is acknowledged that these therapeutic strategies still harbour untapped potential for further advancement. Gaining a comprehensive understanding of the merits and limitations of these treatment modalities holds the promise of offering novel perspectives for clinical practice and foundational research endeavours. In this review, we discussed the different treatment modalities, including small molecule targeted drugs, peptide drugs, antibody drugs, cell therapy, and gene therapy. It will provide a detailed explanation of each method, addressing their status of development, clinical challenges, and potential solutions. The aim is to assist clinicians and researchers in gaining a deeper understanding of these diverse treatment options, enabling them to carry out effective treatment and advance their research more efficiently.
Similar content being viewed by others
Molecular targeted therapy for anticancer treatment
From signalling pathways to targeted therapies: unravelling glioblastoma’s secrets and harnessing two decades of progress
Treatment landscape of triple-negative breast cancer — expanded options, evolving needs
Introduction.
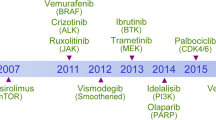
Cancer has become a crucial public health challenge. Daily, over 52,900 individuals are diagnosed with cancer, and more than 27,000 people lose their lives to this disease. 1 It is estimated that by 2040, there will be 28 million new cases and 16.2 million deaths worldwide. 2 The best strategy for continuously reducing global cancer mortality is the widespread implementation of precise and individualized treatment and increased investment in advancing cancer drug research. The cancer treatment timeline documents the evolution of therapies over the past 170 years, highlighting the transformative treatments that have emerged to enhance clinical outcomes and improve patients’ quality of life. Starting from the early use of general anesthesia in surgical resections in the mid-1800s, to Wilhelm Conrad Röntgen’s invention of X-rays at the end of the 19th century, 3 which initiated the era of combining radiation with surgery for cancer treatment, to the breakthroughs in chemotherapy during World War II (WWII), 4 and the recent advancements in immunotherapy and gene therapy, each milestone has played a pivotal role in the ongoing fight against cancer.
In 1990, the U.S. Food and Drug Administration (FDA) approved the use of BCG for the intravesical instillation treatment of superficial bladder cancer. 5 In 1997, the FDA approved Rituximab, the first targeted therapy for B-cell lymphomas, 6 marking the beginning of a new era of targeted treatments. Two years later, Trastuzumab was introduced, becoming the first targeted therapy for breast cancer by targeting the HER2 protein, 7 significantly impacting treatment strategies. The year 2001 saw another milestone with the FDA approval of Imatinib, 8 the first kinase inhibitor, which revolutionized the treatment of chronic myeloid leukemia and other rare gastrointestinal tumors. In 2003, Gefitinib became the first targeted therapy approved for non-small cell lung cancer (NSCLC), 9 followed by Erlotinib in 2004, 10 expanding the options for NSCLC patients. Also in 2004, Bevacizumab 11 was approved as the first “anti-angiogenic” drug, demonstrating a new approach to cancer therapy by targeting the blood supply to tumors. That same year, Rigvir 12 was approved in Latvia for melanoma treatment. Subsequent years saw a steady stream of targeted drugs, with Sorafenib 13 in 2005 for renal cell carcinoma. In 2011, Carl June’s successful application of CAR-T cell therapy for leukemia treatment marked a significant step forward in immunotherapy. 14 The 2014 FDA approval of Pembrolizumab and Nivolumab for the treatment of melanoma, along with the accelerated approval of Trametinib and Dabrafenib for patients with BRAF-mutant melanoma, marked a new beginning in cancer immunotherapy. 15 , 16 , 17 Preventive measures also advanced, with the approval of the nine-valent Gardasil 9 vaccine in December 2014, 18 offering broader protection against HPV strains associated with cervical cancer. The approval of T-VEC in 2015 and Delytact in 2021 for melanoma and malignant glioma, respectively, highlighted the resurgence of oncolytic viruses as a cancer treatment modality. 19 , 20 The 2020 s have seen further advancements with the FDA approval of Sotorasib, the first small molecule inhibitor targeting specific KRAS gene mutations. 21 In 2021, the National Comprehensive Cancer Network (NCCN) guidelines highlighted the combination of atezolizumab and bevacizumab as the preferred first-line treatment option for patients with hepatocellular carcinoma (HCC). 22 This recommendation underscores the importance of immunotherapy and anti-angiogenic therapy in the frontline management of this aggressive form of cancer, reflecting the evolving landscape of cancer care and the continuous efforts to improve patient outcomes (Fig. 1 ). As of December 2021, there were 107 operational proton and heavy ion therapy centers worldwide, including 12 carbon ion therapy centers. 23
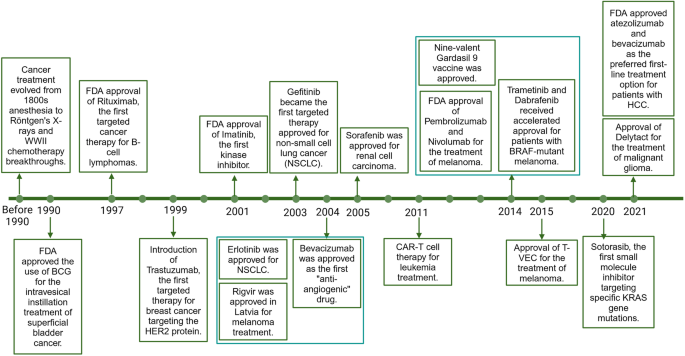
The milestone of cancer therapy development . This timeline illustrates the significant advancements in cancer therapy over the past 170 year. Beginning with the adoption of general anesthesia for surgical procedures in the mid-1800s and the groundbreaking invention of X-rays by Wilhelm Conrad Röntgen in the late 19th century, which paved the way for radiation therapy combined with surgery, the field of oncology has witnessed a series of transformative treatments.Key developments include the introduction of chemotherapy during World War II, the advent of immunotherapy, and the more recent progress in gene therapy. The 1990s marked a turning point with the FDA approval of BCG for bladder cancer treatment and Rituximab for B-cell lymphomas, initiating the era of targeted therapies. Trastuzumab and Imatinib further revolutionized the treatment of breast cancer and chronic myeloid leukemia, respectively. The new millennium brought targeted therapies for non-small cell lung cancer with drugs like Gefitinib and Erlotinib, and the first anti-angiogenic drug, Bevacizumab, which targeted tumor blood supply. Rigvir’s approval in Latvia for melanoma treatment signified the global reach of cancer advancements. The successful application of CAR-T cell therapy by Carl June in 2011 and the FDA approval of Pembrolizumab and Nivolumab in 2014 for melanoma treatment highlighted the potential of immunotherapy. Preventive measures also evolved, as evidenced by the approval of the nine-valent Gardasil 9 vaccine, offering broader protection against HPV strains linked to cervical cancer. The resurgence of oncolytic viruses was evident with the approval of T-VEC and Delytact for melanoma and malignant glioma, respectively. The 2020 s have introduced targeted therapies for KRAS gene mutations with Sotorasib and the combination of atezolizumab and bevacizumab as a preferred first-line treatment for hepatocellular carcinoma, as endorsed by the NCCN guidelines in 2021. This figure encapsulates the continuous innovation and dedication to enhancing cancer care, reflecting the dynamic nature of the fight against cancer and the pursuit of improved patient outcomes. This figure was created with Biorender.com
Now, the field of oncology is experiencing a proliferation of tumor drugs, leading to a flourishing phase in the oncology drug market. According to Frost & Sullivan, the global anti-tumor drugs market is expected to grow at a CAGR of 4.20% from 2022 to 2029, with an estimated market size of USD 94,340 million in 2022 and projected to reach USD 125,825.86 million by 2029. 24 With the thriving development of tumor basic research in cell targets, signaling pathways, immune escape, etc., there are several trends in the development of tumor drugs. Firstly, the functions of tumor drugs are becoming increasingly specialized. Tumor drugs are no longer limited to chemotherapy-based tumor killing, such as Alkylating agents (e.g., Mechlorethamine, Cyclophosphamide), 25 but also include differentiation in angiogenesis inhibition (e.g., EGFR inhibitors like Lapatinib and Gefitinib and VEGFR inhibitors e.g., Sunitinib, Sorafenib), 25 tumor metabolism regulation (e.g., IDO1 inhibitors, IDH1 and IDH2 inhibitors), 26 and restoration of self-immunity (e.g., anti-CD20 antibodies (rituximab and obinutuzumab). 27 The combination of various drugs makes tumor therapy more precise and effective. Secondly, there is a gradual shift in tumor drugs from small molecules to large molecules. The evolution includes small molecule inhibitors (e.g., Imatinib, Lapatinib, and Neratinib), peptides (including Sandostatin, Lutathera, Kyprolis, and Zoladex), antibody drugs (e.g., trastuzumab deruxtecan [Enhertu] and Trastuzumab emtansine [T-DM1]), and cell therapies (comprising various chimeric antigen receptor [CAR]-T cells, NK cells, macrophages, and tumor-infiltrating lymphocyte [TIL] therapy). The design and preparation of tumor drugs have become more complex, and the preparation methods have advanced. Thirdly, tumor drugs are shifting from inhibiting tumor cell functions (e.g., tumor neoantigen suppression, surface receptor inhibition) to regulating self-immune activation, such as with Antibody-Drug Conjugates (ADCs). Fourthly, the treatment landscape has expanded from monotherapy to combination therapies, encompassing a variety of immunomodulators, anti-angiogenic drugs, chemotherapies, and targeted therapies. Lastly, a diverse array of treatments, including previously ‘undruggable’ targets, peptide drugs, monoclonal antibodies, ADCs, cell therapies, gene therapy, neoantigen and cancer vaccines, oncolytic viruses, immunologic adjuvants, innate immunity activators, proton therapy, carbon ion therapy, photothermal and photodynamic therapy, and anti-angiogenesis therapy, is reshaping the old cancer drug market and ushering in a more diversified era of tumor treatment.
In this review, we will introduce different types of anti-tumor drugs based on the principles of drug classification. We will analyze these different anti-tumor drug strategies from the perspective of their mechanisms of action, development history, basic design principles, advantages, disadvantages, and current existing bottlenecks. We aim to contribute to the translation of basic scientific research into clinical drug selection.
Small molecule inhibitors
Small molecule inhibitors can interfere with or block the activity of specific molecules by interacting with them. These molecules are typically proteins, which play important roles in cell signaling, gene expression, and metabolism. By binding to target molecules, small molecule inhibitors can disrupt their normal function, thereby interfering with disease progression or treating certain conditions.
Artificial intelligence and cryo-EM for protein structure analysis
Computational modeling has become an important tool in small molecule drug discovery, enabling faster and more successful identification of drug candidates. Computational methods are applied at three main stages of small molecule drug discovery, including the initial identification of active substances (i.e. lead compound discovery) through large-scale exploration of chemical space and the use of molecular docking, hit-to-lead compound selection using machine learning and physics-based methods to refine lead compounds, and multi-parameter optimization of lead compounds using physics-based, structure-based, QSAR, and machine learning methods to achieve the desired target product features. 28 These methods allow for a balance of potency, selectivity, and ADMET properties to support the efficacy relationship required for in vivo pharmacokinetics/pharmacodynamics under tolerable exposure. By integrating computational modeling with experimental validation, more efficient and successful drug discovery can be achieved.
In the step of initial identification of active substances, structure biology is crucial for small molecular inhibitor discovery. With the availability of atomic resolution data on the active or regulatory sites of proteins, the design of drug structures becomes feasible. At present, three main techniques are used in structural biology research: X-ray crystallography, nuclear magnetic resonance (NMR), and cryo-EM. 29 X-ray crystallography reveals atomic details for small, crystalline complexes up to 150 kD but is limited for larger or membrane proteins. 30 NMR can analyze smaller proteins (up to 50 kD) in solution without crystals, needing isotope labeling. 31 Cryo-EM determines structures of large complexes and membrane proteins without crystallization, capturing various conformations for easier structural analysis, which is highly convenient for elucidating structures.
Due to structural analysis using cryo-EM of biologically important macromolecules becomes increasingly complex, Artificial intelligence (AI) and cryo-electron microscopy (cryo-EM) have emerged as powerful tools to solve this problem. The current workflow for cryo-EM combined with AI includes particle identification, three-dimensional reconstruction, resolution enhancement, automated high-throughput analysis, pattern recognition, drug design assistance, and data interpretation as well as hypothesis generation. Since cryo-EM AI processes biological proteins while maintaining their bioactivity, a lower electron dose is used to reduce radiation damage. 32 , 33 , 34 Noisy images with low contrast were generated, making it difficult to discern details from the raw micrographs. With machine learning models, such as DeepPicker, 35 DeepEM, 36 and convolutional neural networks (CNNs), AI are trained to recognize and classify different types of particles. Through deep learning of two-dimensional image data, feature extraction, and classification of key information, AI can construct initial models for the targeted proteins. Further iterative optimization, parameter adjustments—such as particle orientation, position, and scaling—and resolution enhancement lead to the final acquisition of high-resolution 3D protein models suitable for research and drug development. Based on the established models, AI can perform high-throughput data analysis, decipher protein conformations, calculate molecular binding affinities, predict, and explore interactions between small molecules and proteins, and assist in discovering and optimizing potential drug candidates. Finally, by predicting the function of drugs and the generated protein structures, the inhibitory efficacy of small molecule drugs on proteins is evaluated. The application of AI in cryo-EM has significantly increased the speed and quality of data analysis, accelerating the entire process from raw data to final structural determination, and bringing revolutionary changes to the fields of structural biology and drug discovery.
The advantage and disadvantage of small molecular inhibitors
As of 2023, the US FDA has approved 72 small molecule therapeutic protein kinase inhibitors for cancer. 37 There are 57 anti-solid tumor drugs. 37 For example, Imatinib (Glivec), as the first small molecular inhibitor specifically designed to address the mechanisms of tumor formation, has heralded a new epoch in cancer therapy with its successful development and application. Ithas been approved for the treatment of eight different diseases, including certain types of leukemia and gastrointestinal stromal tumors (GIST). 38 Imatinib is a tyrosine kinase inhibitor that targets specific abnormal protein kinases involved in the growth and proliferation of cancer cells. Its approval for multiple indications highlights its efficacy and versatility in treating various types of solid tumors. 38
Compared to other drugs, small molecule drugs have simpler and less expensive synthesis and preparation processes. They are often administered orally, which is more convenient for patients and has fewer side effects. However, small molecule drugs target proteins, typically enzymes or receptors, which may result in limited inhibitory effects on membrane proteins and secretory proteins. Additionally, due to the influence of metabolism, it is challenging to adjust the dosage of small molecule inhibitors to achieve optimal efficacy. In particular, the challenge of undruggable proteins which has hampered the design of drugs that target many oncogenes.
Strategies for undruggable proteins
It has been reported that notable and infamous players in cancer initiation and progression, which are not treatable by conventional therapies, include transcription factors (such as p53, MYC, E2F, or Kruppel-like factor 4 (KLF4)), phosphatases (such as PP2δ, PP2A, or PTP1B), and the well-known RAS family. Despite being identified as the first human mutated cancer gene in 1982, the RAS family has remained undruggable. 39 It wasn’t until 2013 that Shokat first reported the feasibility of using small molecule covalent binding to target the KRAS G12C mutant (one of the most common RAS mutations in non-small cell lung cancer (NSCLC)). 40 New inhibitors targeting the KRASG12C mutation, such as Adagrasib 41 and Sotorasib, 42 have shown clinical efficacy in patients with locally advanced or metastatic NSCLC, and the FDA has approved these drugs for the treatment of patients with KRAS G12C mutated NSCLC. However, not all RAS mutations are G12C, and compounds targeting other KRAS subtypes beyond G12C are also in development, which are commonly found in NSCLC, pancreatic cancer, and colorectal cancer. 43 Compared to RAS, other fusion transcription factors commonly seen with pediatric cancers have been deemed undruggable due to large protein–protein interaction (PPI) interfaces or their lack of deep protein pockets. 44 Based on the various characteristics of existing undruggable proteins, current drug design strategies include covalent modulation, allosteric inhibition, Proteolysis-targeting chimeras (PROTAC) and Molecular Glue Degraders (MGDs). 45
Covalent modulation
Covalent modulation refers to the binding of small molecule inhibitors to their targets through the formation of irreversible covalent bonds (i.e., chemical bonds) to alter their activity and function. The interaction between the drug molecule and the target is highly stable until the covalent bond is broken by specific biochemical processes, such as metabolism. This approach enables drug design for proteins that lack surface pockets. It is important to highlight the KRAS family of genes, which have been synonymous with undruggable targets. KRAS, as a widely acting gene, frequently undergoes mutations in various cancers. The G12C mutation, for instance, accounts for approximately 25% of mutations in non-small cell lung cancer. 46 Normal KRAS protein functions as a GTPase, binding to GTP in its active state and switching back to an inactive state by binding to its hydrolysis product GDP after GTP hydrolysis. 47 KRAS mutations (such as the G12C mutation) reduce the GTPase activity of the KRAS protein, causing it to persistently bind to GTP, thereby continuously activating downstream signaling pathways, such as MAPK/ERK and PI3K/AKT, promoting tumor proliferation. 48 Due to the relatively smooth surface of the KRAS protein, which lacks obvious pockets, and its propensity to bind GTP, the development of drugs that competitively inhibit KRAS binding to GTP has been challenging. Moreover, the development of drugs targeting downstream signaling molecules such as RAF, MEK, ERK, and PI3K has also encountered significant difficulties. 49 Research on KRAS-related inhibitors has been on hold. AMG 510 (Sotorasib), approved by the FDA in 2021, is the first small molecular inhibitor targeting specific KRAS gene mutations. 50 It leverages the unique chemical properties of the cysteine (Cys) residue in the KRAS G12C mutant to covalently bind to this residue, locking KRAS G12C in an inactive state and preventing it from binding to GTP, thereby inhibiting KRAS-mediated downstream signaling pathways and suppressing tumor growth. 51 The launch of AMG 510 signifies a milestone in the “undruggable” proteins’ history. Currently, drugs produced using covalent binding methods target both EGFR and P53. These include EGFR inhibitors Afatinib, Dacomitinib, and Osimertinib, as well as P53 inhibitor KG13. Clinical trials for other targets are also underway.
Allosteric inhibition
Allosteric inhibition refers to inhibitors bind to allosteric sites on proteins to change the protein’s conformation, thereby altering its biological function. Allosteric modulation is prevalent in various intricate cellular activities, such as Signal Transduction, Enzymatic Catalysis, Cellular Metabolism, and Gene Regulation. 52 G protein-coupled receptors (GPCRs) and protein kinases are two large classes of molecules related to numerous cellular activities. For example, Asciminib 53 (ABL001) is an allosteric inhibitor for chronic myeloid leukemia (CML) that locks BCR-ABL1 in an inactive conformation by binding to the myristoyl pocket (STAMP), overcoming drug-resistant mutations such as the T315I mutation. Cobimetinib is a MEK kinase inhibitor, used for treating melanoma with BRAF V600E or V600K mutations. 54 SHP099 is an allosteric inhibitor that inhibits SHP2’s activity by binding to multiple structural domain interfaces of SHP2. In terms of design, allosteric modulator target high-entropy, low-conservation allosteric sites, and their significant variability determines that the corresponding allosteric drugs have higher selectivity. 55 Unlike traditional inhibitors, which rely on competitive occupancy, these protein-protein interactions drugs bind with a transient nature, allowing low doses of the medication to achieve the desired effect and exhibiting greater resistance to drug resistance. However, allosteric inhibitors also face challenges. Firstly, allosteric inhibition is highly dependent on protein model analysis, thus requiring increased computational power and algorithmic improvements to assist in identifying allosteric sites. Secondly, drug-resistant mutations and species differences in animal models and human may cause changes in the binding of allosteric inhibitors to their sites, requiring further in-depth research to circumvent this issue.
PROTACs are a strategy that utilizes protein degradation mechanisms to eliminate target proteins by simultaneously connecting the target protein with an E3 ubiquitin ligase to form a ternary complex molecule for the degradation of the target protein. The development of anticancer PROTACs primarily uses ligands for E3 ligases such as CRBN, VHL, MDM2, IAPs, AhR, DCAF15, DCAF16, RNF4, and RNF114. 56 , 57 , 58 , 59 , 60 , 61 , 62 These ligands are tumor-specific to prevent off-target toxicity of PROTACs. Currently, PROTACs have been developed for various targets to combat solid tumors and malignant hematological cancers, such as those targeting AR (Bavdegalutamide 63 (also known as ARV-110), CC-94676, 64 AC176, 65 HP518, 66 and ARV-766 67 ), ER (ARV-471 68 and AC682 69 ), and BTK (NX-2127, 70 NX-5948, 71 BGB-16673, 72 and HSK29116 73 ). These drugs have entered clinical trial stages, with Bavdegalutamide (NCT03888612), ARV-471 (NCT04072952), and NX-2127 (NCT04830137) showing their therapeutic effectiveness; the PROTAC targeting MAP4K1 has the potential to be a “first-in-class” therapy mimicking PD-1/PD-L1 targeted therapy; while PROTACs targeting BCL-XL 74 and ALK have shown broad-spectrum antitumor activity, effectively killing leukemia and solid tumor cells in both in vitro and in vivo preclinical experiments. Like allosteric inhibition, PROTACs also showed the advantages of low dose, safety and resistance to drug resistance. However, the current size of PROTACs exceeds 1000 Daltons, making tissue and cellular permeability remain as major challenges.
MGDs are a class of small molecules that facilitate the interaction between target proteins and E3 ubiquitin ligases, leading to the ubiquitination and subsequent degradation of them. They offer potential advantages over PROTACs, such as improved pharmacokinetic properties, including better membrane permeability, cellular uptake, and blood-brain barrier penetration. Despite their promise, the development of MGDs is largely serendipitous and lacks systematic design approaches, necessitating further theoretical exploration to aid in the rational design of these drugs. Notably, the FDA has greenlit clinical trials for several molecular glue agents, such as MRT-2359 (NCT05546268), a GSPT1-targeting molecular glue for multiple solid tumors, SP-3164 (NCT05979857) for its anti-tumor activity in follicular lymphoma models with expected clinical trials in 2023, and IK-595 (NCT06270082) for advanced solid tumors with RAS-MAPK pathway alterations, set to begin in 2024. 75 , 76 , 77
With the development of the aforementioned technologies, proteins that were once considered “undruggable” are gradually shedding that label and becoming “yet-to-be-drugged” proteins. The application of small molecule inhibitors has been further expanded. However, the future of small molecule inhibitors still faces many challenges. For instance, patients with EGFR mutations treated with first and second-generation EGFR tyrosine kinase inhibitors (such as Gefitinib, Erlotinib, Afatinib, and Dacomitinib) approximately have a 50–60% chance of developing resistance within one year of treatment. 78 It is necessary to continue treating patients by replacing other therapeutic methods. Next is the challenge of designing small molecule inhibitors, including the need for further breakthroughs when dealing with highly homologous protein families and “undruggable” targets. Finally, there is the consideration of the safe dosage and metabolic stability of small molecule inhibitors in the body. It is necessary to ensure that small molecule inhibitors are safe and effective within their therapeutic window.
Peptide drugs
Peptide drugs refer to specific therapeutic peptides synthesized chemically, through genetic recombination, or extraction, typically composed of 10–50 amino acids. During the exploratory period before the 1960s, significant advancements were made in peptide drug development. The successful extraction of insulin in 1921 marked a major milestone, leading to improved symptoms in diabetic patients. 79 The rapid development period from 1960 to 2000 saw revolutionary advancements in peptide synthesis. The invention of solid-phase peptide synthesis (SPPS) by Robert Bruce Merrifield made synthesis more convenient and faster. 80 The 1980s witnessed the emergence of recombinant technology and phage display technology, enabling the production of larger molecular weight peptide drugs and the screening of peptides with specific characteristics from large libraries. 81 In the explosive period after 2000, the field of peptide drugs experienced significant growth. Natural peptides were enriched through techniques such as peptidomics from venom and new chemical modification methods. 82 This facilitated the discovery of novel peptide drugs. Additionally, the emergence of novel technologies like multifunctional peptides, constrained peptides, conjugated peptides, oral peptides, long-acting formulations, and delivery systems further advanced the field.
Peptide-based imaging and therapeutic approaches
In addition to their pharmaceutical applications, the high affinity of peptides for specific receptors has developed a specific application in the diagnosis and treatment. For example, peptide Scintigraphy and Peptide Receptor Radionuclide Therapy (PRRT) are two important techniques in neuroendocrine tumors (NETs). Peptide Scintigraphy, as a nuclear medicine imaging technique, uses peptides labeled with radioactive isotopes such as 111In (indium) 83 and 68Ga (gallium), 84 like 111In-octreotide and 68Ga-DOTATOC, to detect tumor cells. These radiopharmaceuticals circulate through the bloodstream to tumor cells that express specific peptide receptors and are imaged using Single Photon Emission Computed Tomography (SPECT) or Positron Emission Tomography (PET). Peptide Scintigraphy is very useful for assessing the staging, distribution, and treatment response of NETs. Peptide Receptor Radionuclide Therapy (PRRT) is a targeted therapy that combines the targeting ability of peptides with the cytotoxicity of radionuclides. Therapeutic peptides are labeled with radionuclides such as 90Y (yttrium) 85 or 177Lu (lutetium), 86 which emit beta particles that are lethal to tumor cells. PRRT is particularly effective for tumors that express somatostatin receptors (SSTRs). Additionally, technetium-99m labeled peptide GX1, which specifically binds to tumor vessels of gastric, colorectal, and glioma tumors, shows promise as a new tumor imaging biomarker. 87 , 88 Copper-64 labeled PD-L1 affinity peptide WL12, 89 with PET imaging results indicating that [64Cu] WL12 can be used as a radiotracer to specifically detect tumors expressing PD-L1, providing a basis for the development of tumor immunotherapy strategies.
The advantage and disadvantage of peptide drugs
As of January 2023, there are approximately 180 peptide drugs on the market globally. 90 International peptide drugs are mainly distributed in diabetes and cancer. In the list of the top 25 best-selling peptide drugs in 2022, four are anti-tumor medications: Sandostatin and Lutathera from Novartis, Kyprolis from Amgen, and Zoladex from AstraZeneca (Table 1 ). 90 , 91 , 92 Compared to small molecule inhibitors, peptide drugs operate at lower concentrations and offer better effects. Classical therapeutic peptides, such as hormones, growth factors, and ion channel ligands, enhance the specificity of peptide cancer therapy by efficiently triggering intracellular effects through specific receptor binding. Compared to other large biomolecule formulations, peptides exhibit lower immunogenicity and are generally safer. They have good tissue penetration, are easily chemically synthesized, and modified, and are cost-effective to produce with high efficacy. However, the drawbacks of peptide drugs are quite evident. Compared to chemical drugs, peptide-based medications have unstable physicochemical properties, shorter half-lives, are easily cleared by the body, and most cannot be administered orally. Chemical modifications, such as acetylation and methylation of the N-terminus, protect peptides from recognition and clearance by proteases or peptidases. The use of synthetic or non-canonical amino acids, such as α-aminobutyric acid and β-amino acids, as well as the design of isomeric amino acid surrogates, are both helpful for increasing their stability. Notably, many peptide drugs are designed for targeting extracellular proteins due to their poor membrane permeability. To improve passive membrane permeability, chemical modifications like peptide cyclization, N-methylation, and the formation of intramolecular H-bonds, along with novel prodrug strategies, are introduced into peptide drug design. Additionally, peptides linked to ligands of membrane receptors can be increasingly absorbed via active membrane transport. Low oral bioactivity is also a primary obstacle for their therapeutic application. To avoid cleavage in the intestinal tract, combining peptides with several protease inhibitors, as well as with permeation enhancers such as citric acid and ethylenediaminetetraacetic acid (EDTA), can increase the efficiency of oral delivery. To decrease renal clearance and extend the half-life of peptides, the molecule’s size is typically enlarged through binding with plasma proteins, such as albumins. 93
Monoclonal antibody therapy
Monoclonal antibodies (mAbs) are produced by B cells and specifically target antigens. There are five isotypes of mAbs: IgG, IgA, IgM, IgD, and IgE. Because of its extended half-life and high affinity, IgG, —particularly IgG1 and IgG4,isotypes are frequently used in the development of monoclonal antibodies. Since the introduction of the first monoclonal antibody drug, Rituximab, in 1997, immunoglobulins have been potent drugs for cancer treatment in recent decades. 94 By 2023, the US FDA has approved 79 therapeutic monoclonal antibodies, of which at least 48 are used for cancer treatment, as summarized in Supplementary Table 1 . 95 These monoclonal antibodies are a class of proteins that target specific antigens to exert single or multiple effects for eliminating cancers.
Ligands or receptors blockades
The anti-tumor effects of representative receptor blockades, such as trastuzumab (Herceptin) and cetuximab (Erbitux), are primarily achieved by directly interacting with the tumor surface receptors HER-2 and EGFR, respectively. This significantly inhibits the progression and migration of cancer, especially in cases of HER2+ breast cancer (Herceptin), 96 metastatic colorectal cancer (Eribitux), and advanced head and neck cancer (Erbitux). 97 Two additional HER-2-targeting monoclonal antibodies, margetuximab (Margenza) and pertuzumab (Perjeta), have been approved for the treatment of metastatic HER-2+ breast cancer, either in combination with or as an alternative to Herceptin. 98 , 99
Ligand blockades, such as bevacizumab (Avastin), work by preventing VEGF-A from binding to its receptors, thereby suppressing angiogenesis and neovascularization. As a first-line anti-angiogenic therapy, Avastin has been approved for treating several types of cancer, including colorectal, lung, and ovarian malignancies. 11 , 100
Cytotoxic mAbs
This cytotoxic monoclonal antibody works by targeting antigens, a strategy frequently used in the treatment of hematological tumors. In 1997, the first mAb licensed for the treatment of B-cell lymphoma was rituximab, also known as Rituxan. It induces B cells to become cytotoxic and initiates caspase-independent programmed cell death by directly blocking the CD20 antigen on B lymphocytes. Rituximab-treated B-cell lymphomas have shown improved overall survival (OS) and progression-free survival (PFS). Compared to 56% for rituximab, the objective response rate for ibritumomab tiuxetan (Zevalin), a CD20-targeting radio-conjugate, was higher at 80%. 101
Additionally, cytotoxic antibodies that directly against CD52 (alemtuzumab), CD47, HLA-DR, CD74, and CD99, without the need for caspase, also lead to the death of target cells. Notably, in multicenter clinical studies, the CD52-targeting monoclonal antibody alemtuzumab demonstrated an extended median OS (12–35.8 months), a median PFS (4.7–19.6 months), and an overall response rate (ORR)(31–54%). 102 Oncology treatment involving monoclonal antibodies that target CD47, HLA-DR, CD74, and CD99 are still under investigated.
Besides, clinical trials are underway to investigate hhow novel cytotoxic mAbs activate apoptosis-related receptors, such as TNF-related apoptosis inducing-ligand (TRAIL) death receptors DR4 and DR5, leading to Fas- and caspase-dependent cell apoptosis. 103
Immune checkpoints blockades (ICIs)
In addition to directly inhibiting tumor antigens, it’s critical to modify anti-tumor immunity. Tumor cells can trick the immune system by abnormally activating inhibitory signals that weaken cytotoxic T cells, leading to a state of immune tolerance and exhaustion. This process has become a promising approach for developing new cancer therapies.
PD-1 (Programmed Death 1) and PD-L1 (Programmed Death-Ligand 1) ICIs are a type of cancer treatment that enhances the immune system’s ability to fight cancer by blocking the proteins cancer cells use to evade immune cells. PD-L1 is a protein that can be upregulated on cancer cells, interacting with PD-1 to effectively ‘instruct’ T cells to stand down and not attack the cancer cells. Notably, Pembrolizumab, Nivolumab, and Cemiplimab are representative anti-PD-1 agents, 104 while Atezolizumab, Avelumab, and Durvalumab are examples of anti-PD-L1 agents. 105 The use of these drugs has been associated with improved outcomes in non-small cell lung cancer (NSCLC), melanoma, renal cell carcinoma (RCC), and other cancers. This progress marks a significant breakthrough in cancer therapy due to the development of PD-1 and PD-L1 ICIs.
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), highly expressed in T cells, is an immune inhibitory molecule that interacts with B7 family of ligands (CD80 and CD86) to induce its immunosuppressive effect. In cytotoxic T cells, CTLA-4 competitively shatters the bond between ligands and its co-receptor CD28, thereby destroying its anti-tumor function. Ipilimumab was approved as an anti-CTLA-4 monoclonal antibody (2011) for interrupting B27/CTLA-4 binding andrestoring T lymphocytes cytotoxicity. 106 In a HIMALAYA clinical trial, tremelimumab (Imjudo), a CTLA4-targeting monoclonal antibody, coupled with durvalumab led to a longer OS (16.43 months) for liver cancer patients compared to 13.8 months for the sorafenib-treated group. 107
T cell immunoglobulin and mucin-domain-containing 3 (TIM-3), lymphocyte activation gene-3 (LAG-3), and T cell immunoglobulin and ITIM domain (TIGIT) represent additional significant immune checkpoint inhibitors (ICIs) for monoclonal antibodies (mAbs). TIM-3, a transmembrane protein, expressed on the surface of various immune cells, including T cells, macrophages, and dendritic cells. It plays a role in the immune response to intracellular pathogens and has been linked to the suppression of Th1 and pro-inflammatory responses. TIM-3 is one of the most extensively studied immunotherapeutic targets to date; however, no drugs targeting TIM-3 have been marketed internationally or domestically. Novartis’ BMS-986207 108 is in Phase 3 clinical trials, while Bristol Myers Squibb’s BMS-986258 109 and Incyte/Agenus’ INCAGN2390 110 are in Phase 2 clinical trials. In China, Hengrui’s SHR-11702 111 and BeiGene’s BGB-A425 112 are in Phase 1 clinical trials, and other companies such as Fulong Hanlin and Fanenshi are still in the preclinical stage.
LAG-3 is another immune checkpoint molecule that is upregulated on activated T cells. It binds to MHC class II molecules on antigen-presenting cells (APCs) and transmits inhibitory signals that reduce T cell responses. Similar to PD-1 and CTLA-4, LAG-3 is overexpressed on exhausted T cells within the tumor microenvironment, aiding in cancer cells’ immune evasion. At least 16 drugs targeting the LAG-3 molecule have entered clinical research worldwide, with BMS’s BMS-986016 being the most advanced. 113
TIGIT is primarily expressed on the surface of T cells and NK cells, and CD155 on tumor cells is a high-affinity ligand for TIGIT. TIGIT can inhibit immune activation by binding to CD155. Roche’s Tiragolumab and BMS’s BMS-986207 114 are examples of independently developed antibody drugs targeting TIGIT, which are still undergoing clinical trials. Additionally, we have listed other ICIs that are currently on the market and their therapeutic effects in patients.
CD24, an emerging class of cancer immunotherapies that modulate immune responses within the tumor microenvironment by targeting the CD24 protein. CD24 is a cell surface molecule that is highly expressed on various cancer cells but has low expression on normal cells. It binds to the Siglec-10 receptor on macrophages, sending a “do not eat me” signal that helps tumor cells evade immune system surveillance and clearance. 115 By blocking this signaling pathway, anti-CD24 monoclonal antibodies can enhance the phagocytic activity of macrophages against tumor cells and potentially activate a broader immune response. Several drugs targeting CD24 are currently in development. IMM47, developed by YiMab, is a pioneering anti-CD24 monoclonal antibody in China for clinical trials, showing tumor eradication in preclinical studies. 116 Pheast Therapeutics, founded by a key figure in CD24 research, has raised substantial funds for drug development. 117 OncoC4 advances a varied pipeline, including CAR-T and bispecific antibodies, towards clinical trials. 118 These drugs target the CD24 protein to enhance immune responses against tumors.
The advantages, disadvantages and challenges of mAbs
In contrast to traditional therapies like chemotherapy and radiotherapy, which are effective in killing tumor cells but also cause damage to normal cells, monoclonal antibodies therapy targets cancer cells with high specificity and reduced toxicity to health cells. Several benefits have been highlighted for mAbs therapy in treating cancers. Firstly, monoclonal therapeutic antibodies are engineered to target specific antigens or proteins with high specificity and affinity, which allows for increased effectiveness and more precise targeted treatment for patients. Secondly, due to their high specific mode of action, monoclonal antibodies pose a lower risk of side effects on health cells. Thirdly, mAbs are fast-responders, and their therapeutic effects could be observed within a short time. Last but not least, several mAbs are immune modifiers, sustaining the long-term activity of immune cells. This activated immune response might persist beyond the duration of treatment, offering long-term therapeutic protection against relapse.
However, mAbs also face several challenges and limitations. The first challenge pertains to high production costs. Monoclonal immunoglobulins are large (~150 kDa) multimeric proteins that contain numerous disulfide bonds and N-linked glycosylation sites, requiring complicated eukaryotic machinery for mass production with high purity in vitro. Large amounts of mAbs are required to achieve clinical efficacy, leading to high productions costs. Alternatively, other production systems, like microorganisms and plants, are under evaluation to lower the cost. 119 , 120
Another unavoidable drawback for therapeutic drugs, including mAbs, is antibody-related side effect. The causes of these sides effects stem from following mechanisms: inherent immunogenicity, suppressive effects on other cells, and over- or long-term activation of immune system. Numerous strategies have been devised to mitigate these side effects. For example, the first therapeutic mAbs are derived from murine system, resulting in poor immune response and a plethora ofside effects. Substitution with a fully human Fc fragment or alternative engineered formats addresses immunogenic inaccessibility. Several mAbs, such as trastuzumab, rituximab, daratumumab, target specific proteins and elicit antibody-dependent cytotoxicity like complement (e.g., C1q) system activation or cell-mediated cytotoxicity via Fc domain interaction with Fcγ receptors (FcγR) on effector cells. 120 This additional immune activation enhances therapeutic effectiveness and extends cytotoxicity. Exploring alternative antibody isotype mAbs, like panitumumab, an anti-EGFR IgG2 antibody for treating colon cancer, and engineering the Fc region through mutagenesis, such as producing defucosylated mAbs, present potential approaches to augment Fc affinity for its Fc receptors, hence diminishing antibody-dependent cytotoxicity. 121
Importantly, low penetration efficiency and long half-life remain a challenge for therapeutic mAbs. It’s reported that no more than 20% administered dose may penetrate the target sites, with the majority remaining in the bloodstream. The remaining antibodies could interact with various types of cells including endothelial cells, monocytes, barrier sites through binding with its Fc-neonatal Fc receptor (FcRn). An extended serum half-life is associated with increased risks of immune-related adverse effects. The large molecule size of mAbs limits tumor penetration and reduces the rate of renal clearance. Besides, FcRn helps to extend the half-life of mAbs and protects them from the clearance of lysosome. Novel antibody engineering technologies are designed to overcome these shortcomings. For example, scFv fragment was developed with short half-life for imaging; 122 medium size (~60 kDa) Diabodies have demonstrated rapid tumor uptake and clearance, suitable for imaging and radioimmunotherapy; 123 and small antibody fragment, such as single variable domains or chemically modified antibodies e.g., anti-TNFα PEGylated Fab fragment 124 or fusion antibody fragments with peptides, 125 are under investigation. Other engineering methods, including fusions with effector proteins, bispecific antibodies, and intrabodies, hold promise for achieving the desired therapeutic benefits while minimizing side effects.
Antibody drug conjugates (ADCs)
Antibody-drug conjugates (ADCs) are a unique class of drugs formed by conjugating monoclonal antibodies, which specifically target antigens, with cytotoxic small molecule drugs (Fig. 2a ). This innovative system is often referred to as a “biological missile” as it mimics the concept of a missile, where the antibody serves as the “guidance system” and the toxin acts as the “warhead”. This allows for precise and targeted “strikes” on specific tissue targets within the human body. Once the ADC drug enters the bloodstream, the antibody component recognizes and binds to the specific antigen present on the target cell. Subsequently, the ADC-antigen complex is internalized by the target cell through endocytosis. Within the cell, the toxin is released after being degraded by lysosomes, ultimately leading to the apoptosis of the target cell (Fig. 2b ).
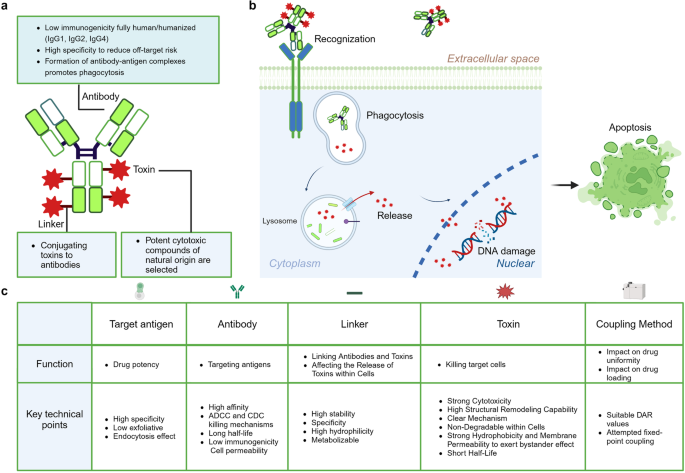
Composition of ADCs and Mechanism of Action in Targeting Cells. a Composition of ADCs. ADCs are composed of an antibody, a linker, and a toxin. The antibody serves as the backbone of the ADC, required to conjugate with the other two components for specific targeted endocytosis within the human body. Therefore, the demands for this part include low immunogenicity and targeting specificity. The linker is the component within the system that connects the antibody to the toxin. The toxin, the element that ultimately kills the tumor, is attached to the linker and travels with the antibody to the target site to exert its effect. b Mechanism of Action of ADCs Targeting Cells. ADCs exert their therapeutic effects through a process that begins with the specific binding of the antibody component of the ADC to antigens on the surface of tumor cells. Upon binding, the ADCs trigger endocytosis, a cellular process where large molecules are internalized into vesicles. These vesicles, known as endosomes, mature by moving through the cell and eventually fuse with lysosomes, where the ADCs are broken down. The cytotoxic drug component, which is linked to the antibody via a cleavable or non-cleavable linker, is then released into the lysosome or directly into the cytoplasm, where it exerts its toxic effects on the tumor cell, leading to cell death. In some cases, the released drug can also affect neighboring tumor cells through a bystander effect, enhancing the overall therapeutic impact. c The five core elements that influence the effectiveness of ADCs. The table outlines the requirements for various components and synthesis processes of ADC. Initially, the selection of ADC antigen substances must prioritize high specificity and low exfoliative and endocytic effects to ensure targeted action. The antibody, a key component of ADCs, demands high affinity, rapid internalization, low immunogenicity, and reliance on ADCC (Antibody-Dependent Cellular Cytotoxicity) and CDC (Complement-Dependent Cytotoxicity) mechanisms, along with a prolonged half-life for effective targeting and action. The linker should exhibit high stability to prevent rupture during circulation, enable specific release in the target area, and be hydrophilic and degradable for bystander effects. The toxin should have potent cytotoxicity, the ability to undergo structural remodeling, a clear mechanism of action, resistance to degradation within cells, strong hydrophobicity for membrane permeability to induce bystander effects, and a short half-life. The coupling method affects drug uniformity and loading, requiring optimal DAR (Drug-Antibody Ratio) values and the implementation of site-specific conjugation strategies. This figure was created with Biorender.com
Five core elements of ADCs
The effectiveness of ADCs is influenced by five core elements: target antigen, antibody, linker, toxin, and coupling method (Fig. 2c ).
The target antigen for an antibody-drug conjugate (ADC) must possess high specificity to ensure precise targeting of cancer cells rather than normal cells, exhibit low exfoliative properties to minimize shedding of the antigen into the bloodstream, and have effective endocytosis capabilities to facilitate the internalization of the ADC into the cancer cells, thereby enhancing the drug’s therapeutic impact.
As the precision guidance component of antibody-drug conjugates (ADCs), antibodies specifically target and deliver the toxin carrier function. They can recognize tumor cell surface target antigens with high specificity, delivering the payload to the tumor cells and mediating the localization and endocytosis of the antibody-drug conjugate within the tumor cells. The ideal antibody characteristics necessitate prolonged circulation half-life, low immunogenicity, cell permeability, and relies on ADCC (Antibody-Dependent Cellular Cytotoxicity) and CDC (Complement-Dependent Cytotoxicity) killing mechanisms. 126 IgG1 is the primary antibody scaffold utilized in ADCs. These IgG1 antibodies boast a prolonged blood half-life, enhanced FcγR binding efficiency, potent ADCC and CDC effects, and a reduced propensity to form oligomers. 127 Employing humanized or human monoclonal antibodies significantly diminishes immunogenicity and alleviates autoimmune effects. Addressing the issue of antibody endocytosis involves adjusting the antibody’s size to ensure sufficient cellular penetration without jeopardizing its half-life.
The linker must possess high stability, ensuring no rupture during the cycling process, enable specific release in the target area, exhibit high hydrophilicity, and be degradable to exert bystander effects. 128 Linkers can be divided into two main categories based on their performance: cleavable linkers (chemical cleavage linkers, enzyme catalyzed cleavage linkers, photo-cleavable linkers) and non-cleavable linkers (sulfide bond linkers, maleimide bond linkers); cleavable linkers are a prerequisite for exerting bystander killing effect, hence becoming the mainstream trend of ADC linkers. The development directions of ADC linkers are to increase hydrophilicity (reduce ADC clearance rate, broaden compatibility with hydrophobic toxins) and increase the effective payload number on a single linker. 128
The toxin must exhibit strong cytotoxicity, high structural remodeling capability, a clear mechanism, non-degradability within cells, demonstrate strong hydrophobicity, and possess membrane permeability to induce bystander effects, while having a short half-life. 128 The coupling method impacts drug uniformity and loading, necessitating suitable DAR (Drug-Antibody Ratio) values and attempted fixed-point coupling. 128
FDA approved ADC drugs
Currently approved ADC drugs primarily target specific proteins overexpressed on tumor cells. HER2 is the most prominent target for ADC development globally, and Ado-trastuzumab emtansine (Kadcyla), Trastuzumab deruxtecan (Enhertu), and Trastuzumab emtansine (T-DM1), which are designed to target HER2-positive cancer cells, have been approved for use in treating HER2-positive breast cancer and other types of cancer. DS-8201 (Trastuzumab Deruxtecan) is a novel antibody-drug conjugate (ADC) composed of a humanized anti-HER2 antibody and an irinotecan-class chemotherapy drug. It has demonstrated promising antitumor activity in various types of cancer, including breast cancer, gastric cancer, and colorectal cancer. Particularly in patients with breast cancer that exhibits low expression of HER2, DS-8201 has shown a significant improvement in therapeutic outcomes compared to other treatment options. Based on the results of the DESTINY-Breast04 study, the FDA has granted accelerated approval for the use of DS-8201 in the treatment of HER2-low expressing breast cancer. 129 As of March 2024, the FDA has approved a total of 15 ADC drugs and there are over 500 clinical trials in progress. The details of 15 FDA approved ADC drugs as shown in the Table 2 . 130 , 131 , 132 , 133
The advantages, disadvantages, and challenges of ADC drugs
As demonstrated above, the greatest advantage of ADCs lies in their reliance on the specific binding of antigen-antibody targeting. This helps to reduce systemic toxic side effects that are characteristic of chemotherapy treatments. Additionally, unlike conventional monoclonal antibodies, ADCs exert their tumor-killing function through the toxin carried by the linker, which allows for greater design flexibility. Furthermore, ADCs can be tailored to target a variety of cancers based on the selection of antigens and antibodies. Moreover, the design of ADCs permits precise control of the ratio of drug to antibody (DAR, Drug-to-Antibody Ratio), which aids in optimizing therapeutic efficacy and safety.
The main drawbacks of ADCs, such as their low internalization, low efficiency, and target-off toxicity, should be addressed by creating more effective ADCs, such as the introduction of bispecific ADCs. For example, ZW38, an asymmetric bispecific CD19-directed CD3 T cell engager antibody, has a significantly higher affinity (>30-fold) to CD3 + T cells than CD19 + B cells. It is specifically designed to mediate effective T cell-guided targeting B cell reduction while eliciting a more “controlled” T cell activation compared to blinatumomab, thereby leading to lower toxicity. 134 Another preclinical study has proved that bispecific ADCs for EGFR/c-MET and HER2/PRLR exhibit improved internalization, increased affinity, and decreased toxicity. 135 , 136 Additionally, striking a balance between therapeutic activity within a confined DAR and the toxicity of payloads presents another challenge. Novel approaches to alter payloads with versatile functional groups, such as amine or thiol groups, will provide new perspectives on addressing this issue. 128 Meanwhile, several site-specific conjugation techniques are being developed to generate homogeneous ADCs, which aim to increase efficiency and decrease toxicity. 137
The development of ADCs still faces challenges. Certain types of cancer lack effective neoantigens. For instance, the discovery and validation of neoantigens are time-consuming, and the expression of antigens varies among individuals. Additionally, tumors may alter the expression of their surface antigens to evade the immune system’s attack, which can make it difficult to identify suitable antibodies. Furthermore, ADCs undergo complex metabolic processes. Due to their diverse designs, ADCs lack uniform metabolic properties. Even ADCs targeting the same antigen may exhibit differences in plasma stability, in vivo metabolism, PK/PD relationships, and adverse reactions owing to variations in antigen epitope recognition, linker sites, coupling chemistry, and the choice of small molecule toxins. 138 , 139 Additionally, ADCs still have toxicities, such as on-target/off-tumor toxicity and off-target/off-tumor toxicity, with the latter being caused by the premature release of toxins into the bloodstream, non-tumor tissues, or the tumor microenvironment. 140 Furthermore, the mechanisms of ADC resistance have not been thoroughly studied, and the production and quality control of ADCs also pose difficulties, all of which affect the production and clinical application of ADC drugs.
Cell therapy
Cell therapy, also known as cellular therapy, is a cutting-edge approach in medicine that utilizes living cells to against cancers. Ongoing research and advancements in cell therapy continue to pave the way for revolutionary breakthroughs in healthcare, providing hope for patients in need of novel and effective treatment options. Currently, popular tumor cell therapies include Chimeric Antigen Receptor T-Cell Therapy (CAR-T), T-Cell Receptor Modified T cells (TCR-T), Tumor-Infiltrating Lymphocytes (TIL), Chimeric Antigen Receptor-Modified Natural Killer (CAR-NK) cells, T-Cell Receptor Modified T-Cells (TCR-T) and Chimeric Antigen Receptor-Modified Macrophages (CAR-M).
The treatment principle of CAR-T involves extracting the patient’s own T cells, using gene editing technology to transfect the chimeric antigen receptor (CAR) gene, expanding the modified T cells in vitro, and then infusing them back into the patient. The CAR structure consists of three distinct domains: the extracellular domain, the transmembrane domain, and the intracellular domain. The extracellular domain of CAR includes the antigen recognition domain, also known as the single-chain variable fragment (scFv), and the hinge region. The scFv is composed of the variable regions of both the light and heavy chains of an antibody, connected by a peptide linker. CARs are being developed to target various tumor-associated antigens such as CD19, CD20, CD22. 141 The hinge region connects the scFv to the transmembrane domain. The transmembrane domain serves as a link between the extracellular domain and the intracellular signaling domain of the CAR. Commonly used transmembrane domains are derived from proteins such as CD4, CD8α, CD28, and CD3ζ (Fig. 3a ). 141
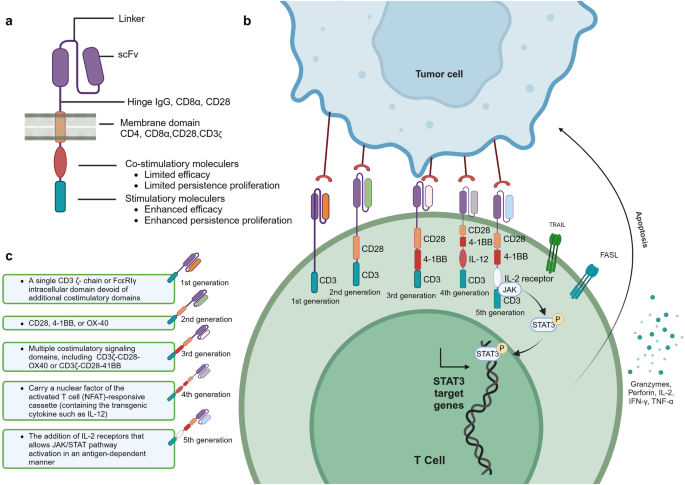
The composition and generation of CAR-T. a Illustrates the structure and mechanism of action of a Chimeric Antigen Receptor (CAR). The CAR is composed of three main domains: the extracellular domain, which includes the antigen recognition domain (scFv) and the hinge region; the transmembrane domain that anchors the receptor in the cell membrane; and the intracellular domain responsible for signaling. The scFv is engineered to recognize specific tumor-associated antigens, such as CD19, CD20, and CD22. The hinge region allows for flexibility in the CAR’s structure, while the transmembrane domain links the extracellular recognition capabilities to the intracellular signaling pathways. The intracellular domain typically contains co-stimulatory motifs that enhance T cell activation upon antigen recognition. b The therapeutic process of CAR-. Genetically engineered CAR-T cells are infused into the patient, where they specifically recognize and bind to tumor antigens via their CARs. This interaction leads to the activation of the CAR-T cells and the release of cytotoxic molecules, such as perforin and granzyme B, which induce apoptosis in the tumor cells. c The evolution of CAR-T. It outlines the five generations of CAR-T cell development. First-generation CAR-T cells had basic signaling domains but lacked co-stimulatory signals, resulting in limited in vivo proliferation and clinical efficacy. Second-generation CAR-T cells included additional co-stimulatory domains, significantly improving their potency and persistence. Third-generation CAR-T cells further enhanced tumor lysis and cytokine secretion by incorporating dual co-stimulatory molecules. Fourth-generation CAR-T cells were designed with controllable suicide genes and pro-inflammatory cytokines for enhanced solid tumor targeting. Fifth-generation CAR-T cells, or “off-the-shelf” universal CAR-T cells, are created by CRISPR/Cas9 gene editing to generate allogeneic T cells, addressing potential GVHD issues. This figure was created with Biorender.com
The mechanism of CAR-T cell therapy involves several key steps. CAR-T cells are genetically engineered T cells that are designed to target specific antigens present on the surface of tumor cells. Once infused into the patient’s body, CAR-T cells recognize and bind to these tumor antigens through theCAR. This binding triggers the activation of the CAR-T cells, leading to the release of cytotoxic effector molecules such as perforin and granzyme B. These substances directly induce apoptosis, or programmed cell death, in the tumor cells (Fig. 3b ). Furthermore, CAR-T cells can recruit other immune cells, such as natural killer cells and macrophages, to the tumor site. 142 This recruitment is achieved through the secretion of cytokines by the CAR-T cells. The immune cells work together to attack and eliminate the tumor cells. One of the remarkable features of CAR-T cell therapy is the potential to form memory T cells. These memory T cells can persist in the body after the initial treatment and provide long-term protection against the recurrence of tumor cells. This memory response contributes to the sustained anti-tumor effects of CAR-T cell therapy. Understanding the mechanism of CAR-T cell therapy is crucial for its successful application in treating various types of cancer.
The development process of CAR-T has gone through five generations. The first-generation CAR-T cells contained intracellular signaling domains but lacked co-stimulatory molecules. 143 These CAR-T cells had limited proliferation in vivo and were not effective in killing tumor cells on a large scale, resulting in unsatisfactory clinical trial outcomes. The second-generation CAR-T cells added a co-stimulatory domain (CD28, 4–1BB, OX40, etc.) to the intracellular domain. After the single-chain antibody on the extracellular domain recognized the tumor cells and bound to the tumor antigen, the T cells could simultaneously receive antibody stimulation signals and co-stimulatory signals. 144 This made the second-generation CAR-T cells far more potent than the first-generation, with longer survival time and enhanced proliferation and tumor-killing ability. The third-generation CAR-T cells incorporate two co-stimulatory molecules simultaneously to enhance tumor lysis ability and increase cytokine secretion, thereby boosting the killing power against tumors. Common combinations include CD28 + 4–1BB or CD28 + OX40 as dual co-stimulatory molecules. 145 Whether the third-generation CAR-T cells are superior to the second-generation ones still needs to be proven in clinical trials. The design of fourth-generation CAR-T cells involve adding controllable suicide genes and pro-inflammatory cytokines (such as IL-12, IL-15, IL-18) to the CAR structure, which allows for controlled survival time of CAR-T cells in the body and enhances the efficacy in killing solid tumors. 142 The fifth-generation CAR-T cells, known as “off-the-shelf” universal CAR-T cells, are designed to disrupt the TCR genes and HLA class I genes of T cells using CRISPR/Cas9 gene editing (Fig. 3c ). This generates allogeneic universal CAR-T cells and eliminates graft-versus-host disease (GVHD) concerns. 142
The challenges and strategies about CAR-T cell therapy
In addition to the practical challenges of cost issues, limitations in meeting inclusion criteria, and unforeseen challenges during the gap period between leukapheresis and infusion, there are also ongoing technical barriers that need to be overcome in the field of CAR-T therapy (Fig. 4 ).
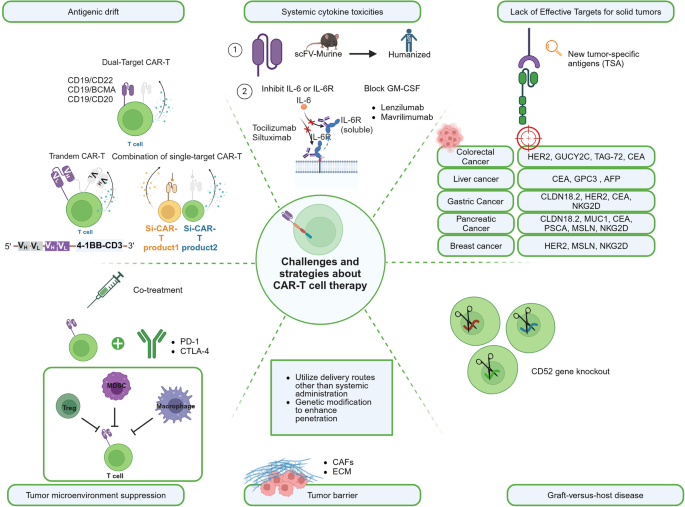
The challenges and strategies about CAR-T cell therapy . The main challenges currently faced in CAR-T therapy include: Antigenic drift, Systemic cytokine toxicities, Lack of effective targets for solid tumors, Tumor microenvironment suppression and epitope expansion, Tumor barrier, Graft-versus-host disease (GVHD), and Host immune rejection, along with their corresponding primary solutions. This figure was created with Biorender.com
Antigenic drift
Antigenic drift is an immunotherapy resistance commonly observed in CAR-T therapy. Although, phase I trials of CD19 CAR T cells in patients with B-cell acute lymphoblastic leukemia (B-ALL) showed response rates between 70% and 90%, there are 7–25% patients resulting in CD19 antigen loss. Other studies demonstrated that loss of BCMA and GPRC5D is observed in 4% and 35% myeloma patients after treatment with BCMA CAR-T and GPRC5D CAR-T, respectively. 146
To overcome these challenges, various strategies have been explored. One approach is the use of dual-target CAR-T, which targets multiple antigens simultaneously. In CD19 CAR-T therapy, it is generally accepted to use a combination of CD19 with CD22, BCMA, and CD20. 147 A bispecific CAR-T (CD19–22.BB.z-CAR) dual-targeting CD19 and CD22 was investigated in a phase I study for patients with large B cell lymphoma (LBCL) and relapsed/refractory B cell acute lymphoblastic leukemia (R/R B-ALL). For both B-ALL and LBCL, the complete response (CR) rates were 29% and 100%, respectively. Minimal residual disease-negative (MRD-) complete remission was attained by 88% of R/R B-ALL patients. Four (29%) patients with LBCL and five (50%) patients with B-ALL exhibited low or negative CD19 expression at the time of progression after the treatment. 148 A similar result was shown in 13 B-ALL patients, where 84.6% of patients achieved CR with dual CD19xCD22 CAR-T cell (SCRI-CAR19x22) therapy, and 95% were MRD-. In three out of the four relapses, CD19 expression remained negative. 149 Aftertreatment with CD19/BCMA dual-targeting Fastcar-T GC012F for patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma (R/R B-NHL), the 3-month overall response rate was 100%, with 77.8% (7/9) reaching CR and no relapse was noted within the follow-up period. 150
Another strategy is tandem CAR-T, where a single CAR construct contains two single-chain variable fragments (scFvs) to target different antigens on one cancer cell surface. In a phase I clinical investigation, by day 28 following therapy with tandem bispecific anti-CD20, anti-CD19 (LV20.19) CAR T cells, 18 (82%) of the R/R B-ALL patients experienced an overall response, with 14 (64%) achieving a CR, and 4 (18%) having a partial response (PR). Remarkably, patients who experienced treatment failure or relapsed did not exhibit loss of the CD19 antigen. 151 In Dai et al.’ study, after receiving another bispecific tandem CD19/CD22 CAR-T therapy, the patients demonstrated a favorable outcome, with a high MRD- complete response rate (100%) reached in B-ALL patients (n = 6); However, one patient relapsed at 5 months post-treatment with decreased CD22 density and negative CD19 expression. 152 Additionally, the combination of different single-target CAR-T therapies is being investigated. These strategies aim to enhance the efficacy and durability of CAR-T therapy and improve outcomes for patients. Sequential administration of two single-target CAR-T therapies, such as the CAR22/19 cocktail CAR-T therapy, was investigated by Huang et al. 153 Among the 50 R/R B-ALL patients, 48 (96%) achieved CR or complete remission with incomplete count recovery (CRi) with 47 (94%) obtaining MRD - CR/CRi. 23 individuals relapsed, but none of them exhibited evidence of CD19 or CD22 antigen loss.
Systemic cytokine toxicities
Upon infusion, CAR-T cells become activated and rapidly proliferate, causing a massive release of cytokines and triggering non-specific inflammatory reactions. These may lead to systemic cytokine toxicities, including cytokine release syndrome (CRS), haemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS), and immune effector cell-associated neurotoxicity syndrome (ICANS). To address these toxicities, one approach is modifying the CAR structure by replacing murine counterparts with humanizing or fully human antibody fragments, aiming to reduce immunogenicity and improve safety. Also, targeting specific cytokines associated with severe CRS, such as IL-6 and IL-1, has been explored [37]. Anti-inflammatory drugs such as tocilizumab and siltuximab can inhibit the action of interleukin-6 (IL-6) and the interleukin-6 receptor (IL-6R), thereby reducing the CAR-T-associated CRS without impairing CAR-T activities or causing T cell apoptosis. 154 , 155 , 156 , 157 , 158 , 159
Inhibiting the inflammatory cascade initiated by GM-CSF (granulocyte-macrophage colony-stimulating factor) has also shown promise in mitigating cytokine toxicities effects. 160 GM-CSF inhibitors such as Lenzilumab and Mavrilimumab can block the action of GM-CSF, thereby reducing the activation of inflammatory cells and the release of inflammatory cytokines. 161
Lack of Effective Targets for solid tumors
Unlike the targets for hematologic malignancies that are mostly single and specific, tumor-specific antigens (TSA) are rare in solid tumors. Common tumor-associated antigen (TAA) targets include CEA, HER2, GPC3, EpCAM, etc. are often expressed on vital organs, which severely limit the application of CAR-T in solid tumors. 162 The continuous discovery of highly expressed and CAR-T-developable targets has led to rapid growth of clinical pipelines, such as GPC3 highly expressed in liver cancer, CLDN18.2 highly expressed in gastric and pancreatic cancer, EGFRvIII in glioblastoma. 163 , 164
Tumor microenvironment suppression and epitope expansion
In solid tumor, immune checkpoint such as PD-1, TIM-3, or CTLA-4 are commonly overexpressed and may cause the exhaustion of CAR-T cells. The tumor microenvironment (TME) contains immunosuppressive cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2 macrophages. These cells release cytokines like transforming growth factor-beta (TGFβ) and interleukin-10 (IL-10) within solid tumors, which diminish the effectiveness of CAR-T cells in fighting tumors. 165 To overcome this, strategies include combining CAR-T cells with immune checkpoint inhibitors such as PD-1 inhibitors in immunotherapy and genetically modifying CAR-T cells to enhance their immune response and resistance to inhibitory factors, thereby improving their anti-tumor activity. 166 Several clinical trials on the combination of CAR-T and immune checkpoints blockades are summarized in Table 3 .
Tumor barrier
The immunosuppressive tumor microenvironment and physical tumor barriers, such as the tumor stroma, can restrict the infiltration and migration of CAR-T cells, limiting their effectiveness in treating solid tumors. One main reason is the lack of relevant receptors on CAR-T cells that match the chemokines secreted by solid tumors, which hinders their homing ability to the tumor site. Strategies include utilizing delivery routes other than systemic administration, such as intrathoracic injection, which has demonstrated superiority in treating malignant pleural mesothelioma. 167 Another approach involves genetic modification of CAR-T cells to enhance their penetration, such as expressing heparinase, an enzyme that degrades HSPG, which can enhance tumor infiltration and anti-tumor activity. 168
Graft-versus-host disease (GVHD) and host immune rejection
Allogeneic CAR-T therapies face two main challenges: graft-versus-host disease (GVHD) and host immune rejection of foreign cells, both of which can limit the effectiveness and persistence of anti-tumor activity. To address these challenges, most allogeneic CAR-T therapies use gene editing to knock out endogenous T cell receptors (TCRs) and other proteins that may trigger host immune rejection. Gene editing tools like TALENs, ZFNs, or CRISPR/Cas9 are commonly used to target the TRAC gene and permanently knock out proteins that elicit immune rejection. 169 However, gene editing also carries potential safety risks such as off-target effects and chromosomal abnormalities. Another strategy involves CD52 gene knockout to provide allogeneic T cells with resistance to lymphocyte depletion. 170 Disrupting the B2M locus can also prevent host immune system destruction of allogeneic cells by preventing the formation of HLA-1 molecules on T cell surfaces and avoiding recognition as foreign entities. 171
CAR-NK involves introducing a CAR into NK cells, enabling them to specifically recognize and eliminate tumor cells. The cytotoxicity mechanisms of CAR-NK cells primarily include the secretion of cytotoxic granules such as Perforin and Granzyme, which directly kill target cells 172 (Fig. 5a ). Additionally, CAR-NK cells can express tumor necrosis factors like FasL and TRAIL, which induce apoptosis in target cells by binding to them. 173 Another mechanism is ADCC, where CAR-NK cells express FcγRIII (CD16) receptors that bind to the Fc region of tumor antigen-specific antibodies. 174 This activation leads to cytotoxicity and mediates the killing of target cells (Fig. 5a ).
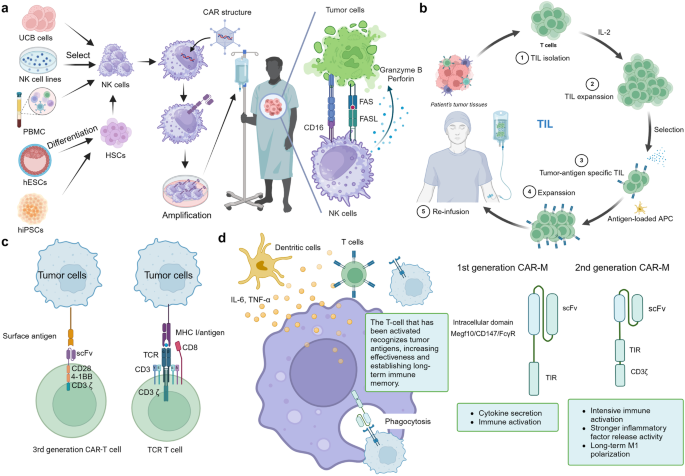
Other kind of cell therapy. a Preparation of CAR-NK and the mechanisms of CAR-NK cell. The preparation of CAR-NK cells involves isolating NK cells from sources such as umbilical cord blood or hematopoietic stem cells, followed by genetic modification to integrate a CAR construct that includes an antigen recognition domain. These cells are then expanded in vitro before being infused back into the patient. Once in the body, CAR-NK cells utilize their CAR to recognize and bind to specific tumor antigens, leading to their activation and the subsequent release of cytotoxic granules and cytokines to eliminate cancer cells. Additionally, the Fc receptor CD16 on NK cells can mediate ADCC, further enhancing their tumoricidal activity. b Preparation of TIL. The preparation of tumor-infiltrating lymphocytes (TIL) involves isolating immune cells from a patient’s tumor tissue, where they have naturally infiltrated. The process typically includes surgical removal of the tumor mass, followed by the mechanical and enzymatic dissociation of the tissue to obtain a single-cell suspension. The cells are then cultured in vitro, and the TILs, which are often CD8+ T cells, are selectively expanded through various techniques, such as interleukin-2 (IL-2) stimulation. Once a sufficient number of TILs are obtained, they are infused back into the patient as part of the adoptive cell transfer therapy, aiming to boost the immune system’s ability to target and destroy cancer cells. c The structure comparation between TCR-T and CAR-T. TCR-T cells are genetically modified to express a specific T-cell receptor that recognizes MHC-presented antigens, allowing them to target a broad range of tumor-associated antigens, including those derived from intracellular proteins. In contrast, CAR-T cells are engineered to express a chimeric antigen receptor that includes an antibody-derived antigen-binding domain, enabling them to target cell surface antigens without MHC restriction. d The overview of CAR-M (workflow and generation). Once inside the patient’s body, the CAR-M cells use their CAR to identify and attach to tumor cells, which triggers the activation of the macrophages, leading to the subsequent engulfment and destruction of the tumor cells. Additionally, they secrete pro-inflammatory cytokines that recruit other cells from the immune microenvironment to join in the attack against the tumor. CAR-Ms are differentiated into first and second generations. The second generation includes an extra CD3 domain compared to the first, endowing it with enhanced pro-inflammatory properties and sustained M1 macrophage activation. This figure was created with Biorender.com
CAR-NK cell therapy offers several advantages over other cell therapies. Firstly, NK cells are widely available from various sources such as NK92 cell lines, PBMC, CD34 + HPC, UCB cells, and iPSC. 175 Among them, NK92 cell lines are commonly used in clinical trials due to their unlimited proliferation capacity in vitro, ease of genetic modification, and no need for patient blood extraction. Secondly, CAR-NK cells are safer than other cell therapies due to their short survival time, reducing damage to non-tumor cells, and lower risk of cytokine release syndrome (CRS), as they produce mainly IFNγ and GM-CSF, which reduces the risk of CRS. 176 Finally, CAR-NK cells are easier to produce for off-the-shelf use, as allogeneic NK cells do not express individual-specific TCR, and the risk of GVHD is much lower than that of allogeneic T cell therapy, making it more suitable for industrialization and large-scale production 177 (Fig. 5a ). Preclinical studies have examined several candidate targets for engineered CAR-NK therapy, including CD19 and CD22 for B-cell malignancies; CD38 and CD138 for multiple myeloma (MM); CD3, CD5, and CD7 for T-cell malignancies; and CD24, EGFR, HER-2 and NKG2D for solid tumors. There are ongoing clinical trials to assess the potential of CAR-NK (Table 4 ). In case of cord blood-derived CAR CD19-NK treatment for B-cell malignancies, one completed clinical trial (NCT03056339) demonstrated a promising efficacy with an ORR of 48.6%, and 1-year OS, and 1-year PFS rate of 68% and 32%, respectively. This suggests CAR-NK as a potential alternative for oncology treatment. 178
TIL therapy
TIL therapy involves isolating TIL cells from tissue near the tumor, amplifying them in vitro with growth factor IL-2, and then reinfusing them into the patient’s body to expand the immune response and treat primary or metastatic tumors (Fig. 5b ). 179 TIL therapy focuses on identifying T cells that effectively target cancer’s specific mutations. After isolating these T cells, which are capable of specifically recognizing the tumor’s mutated cells, they are utilized to mount a cell-mediated immune response. This response primarily involves the direct destruction of tumor cells through the release of cytotoxins.
TIL therapy offers distinct advantages over other cell therapies. Notably, TILs are derived from tumor tissue, unlike the majority of cell therapies which originate from blood. This difference significantly influences the immune cells’ tumor recognition capabilities: over 60% of TILs can identify tumors, in contrast to less than 0.5% of blood-derived immune cells. 180 Furthermore, TILs (Tumor-Infiltrating Lymphocytes) exhibit enhanced specificity due to their well-adapted chemokine receptor systems, which facilitate more effective infiltration into tumor tissues. These TILs are capable of selectively targeting tumor-specific T cells, effectively overcoming tumor heterogeneity without engaging normal tissues. Their high degree of tumor specificity enables TILs to identify and precisely target a range of tumor antigens.
TILs therapy is now being investigated as a potential second-line treatment for a number of malignancies, most notably advanced/metastatic melanoma. Concluding from a meta-analysis involving 13 trials (published from 1988 to 2016), TIL+ recombinant IL-2 treatment for metastatic melanoma, excluding uveal melanoma, was found to have an ORR of 41% and an overall complete response rate (CRR) of 12%. The ORR for the high-dose IL-2 group was 43%, while for the low-dose group was 35%. 181 In a phase 2 clinical trial, TIL treatment was effectively used to treat uveal melanoma; 35% (7/20) of patients showed an ORR, with 1 patient obtaining CR and 6 patients achieving PR. 182 In a recent clinical trial, TIL in combination with high-dose IL-2 treatment for metastatic melanoma demonstrated an 80% disease control rate and a 36% ORR. Surprisingly, in this trial, patients with immunotherapy-refractory melanoma achieved an ORR and DCR of 41% and 81% respectively, suggesting that TIL therapy may offer a viable alternative for treating anti-PD-1 failure. 183 A multi-center, randomized, Phase 3 clinical trial compared TIL therapy with anti-PD-1 immunotherapy in patients with metastatic melanoma. The results showed that patients receiving TIL therapy had better ORR (49% vs. 21%), longer OS (25.8 months vs. 18.9 months), and longer PFS (7.2 months compared to 3.1 months for ipilimumab). 184 These findings suggested that for patients with advanced melanoma, TIL treatment may be more effective than immunotherapy. Future research should focus more on the complexity and variability of TIL preparations and assess the efficacy of TIL therapy in combination with immune checkpoint blockades.
TCR-T is a gene editing technology that introduces the genes of T-cell receptors (TCRs) into the patient’s own T cells. This enables the expression of exogenous TCRs that can specifically recognize tumor antigens and have the activity of killing tumor cells. The TCR is a heterodimer composed of two transmembrane peptide chains, an α chain, and a β chain. Each chain contains a constant region and a variable region. The variable region recognizes and binds to antigen-presenting MHC molecules. 185 TCRs bind with CD3 to form the TCR-CD3 complex, which activates T cells by recognizing and binding antigens presented by MHC molecules, promoting T-cell proliferation and differentiation 186 (Fig. 3b ).
CAR-T therapy uses artificially designed single-chain antibody fragments (CARs) to recognize antigens only on the surface of tumors and activate T cells through intracellular co-stimulatory molecules. TCR-T therapy, on the other hand, can recognize both surface and internal antigens of tumors, making it more suitable for solid tumor treatment. TCR-T therapy relies on affinity-optimized or naturally occurring TCRs to recognize antigens presented by tumor MHC molecules, transmitting stimulating signals through the TCR-CD3 complex. TCR-T therapy possesses the capability to recognize an extensive repertoire of hundreds or even thousands of intratumoral antigens, rendering it highly potent against solid tumors (Fig. 5c ). Moreover, TCR-T cell therapy closely mimics the functionality of endogenous T cells in the human body.
Tumor-associated antigen (TAA), including tissue differentiation antigen (TDA), cancer germline antigen (CGA), and tumor-specific antigen (TSA), which comprise mutation-associated neoantigen, viral antigen and alternative tumor-specific antigens, represent two major classes of targets for TCR-T targets. The treatment with TDA-targeting TCR-T cell therapy has shown disappointing preliminary outcomes in multiple clinical trials, with an ORR of less than 30%. 187 In a Phase 2 clinical trial using MAGE-A4-targeting TCR-T cell treatment for advanced solid tumors, encouraging findings were reported, including a median OS of 15.4 months, a median PFS of 3.8 months, and an estimated 1-year OS rate of 90%. 188 Clinical trials continue to evaluate additional therapeutic targets, such as TSA, neoantigen, or viral antigen. Published data demonstrated that 50% of patients in a clinical trial using viral protein HPV-16 E7 TCR-T cell treatment for metastatic HPV-associated cancers had a positive response. 189 In addition, results from eight ongoing clinical trials investigating the combination of anti-PD-1/anti-PD-L1 and TCR-T cell treatment for metastatic solid tumors are not yet available.
With the advancement in macrophage-based scientific research, CAR-M has emerged as a new direction in cellular therapy. A key distinction between macrophages and T cells is that macrophages are the first responders in the body’s defense against viral infections. In 2018, Dr. Saar Gill and Dr. Michael Klichinsky, CAR-T cell therapy experts from the University of Pennsylvania, founded Carisma Therapeutics with a focus on developing CAR-M therapy for cancer treatment. 190 In 2020, they published a paper in the journal Nature Biotechnology introducing a HER2-targeting CAR-M. In a SKOV3 human ovarian cancer mouse model, CAR-M demonstrated effective tumor killing and extended overall survival in mice. 191 Additionally, CAR-M exhibited the ability to resist TAM-mediated polarization towards M2 macrophages and actively convert them into M1 macrophages. 192 Macrophages recognize and engulf specific cancer cells through CAR while activating downstream inflammatory pathways. This leads to recruitment and activation of antigen-presenting cells and T cells. The body can maintain a long-lasting memory of the killing process to prevent tumor cell resurgence. At present, CAR-M is developing rapidly. The first-generation macrophage CAR uses TIR to replace CD3ζ in CAR-T, enhancing M1 polarization and immune activation. Second-generation CAR-M connects TIR and CD3ζ, leading to strong immunity and extended M1 activation 193 (Fig. 5d ). However, several technical challenges remain to be addressed. Firstly, a significant limitation is the restricted number of cells that can be procured for use. While T cells and NK cells from patients can be expanded in vitro, macrophages do not proliferate in this setting. Recently, Jin Zhang’s team published their findings in Nature Immunology, demonstrating that CAR-iM can be generated from induced pluripotent stem cells (iPSCs) and effectively eliminate tumors through cytoburial mechanisms. 194 , 195 , 196 , 197 This research offers a potential solution to the issue of cell population limitation. Secondly, like CAR-T, CAR-M may also face challenges such as migration and inhibition within the tumor microenvironment, which need further research and investigation.
Clinical trials of CAR-M treatment are being considered for research involving humans. According to recently published research, three patients with malignant peritoneal mesothelioma and ovarian cancer experienced stable disease following intraperitoneal delivery of mesothelin-targeting CAR-M, which was generated from PBMC. 198 In another study, three out of four patients (75%) with HER2-overexpressing solid tumors showed good tolerance and response to treatment with another anti-HER2 CAR M (CT-0508), achieving stablilization of their conditions within an eight-week follow-up. 199 Other two clinical trials NCT05007379 (patients’ organoid derived-CAR-M for breast cancer) and NCT05138458 (PBMC derived-CAR-M for refractory/ relapsed T-cell lymphoma) are still ongoing.
The comparison between different cell therapy
The cellular therapies mentioned utilize diverse technologies and demonstrate their respective potentials in cancer treatment. CAR-T cell therapy is renowned for its significant efficacy in certain hematological cancers but may induce severe cytokine release syndrome (CRS) and has limited effectiveness against solid tumors; CAR-NK cell therapy is considered a potential alternative due to its lower toxicity and CRS risks, yet its clinical application is still in the early stages; TIL therapy leverages immune cells extracted directly from tumors, offering high tumor specificity, but the preparation process is complex and costly; TCR-T therapy can recognize a broad range of tumor antigens, particularly intracellular antigens, but its development and manufacturing are more complex and clinical data are limited; CAR-M therapy enhances the tumor-engulfing capabilities of macrophages and may improve the tumor microenvironment, but as an emerging therapy, its clinical applications and long-term outcomes still require further research and validation. Overall, these cellular therapies in cancer treatment warrant further exploration in the future, while also facing technical challenges, safety concerns, and cost-effectiveness considerations.
Genetic therapy
According to the American Society of Gene and Cell Therapy (ASGCT), gene therapy is described as “the introduction, removal, or modification of a person’s genetic code to treat or cure a disease.” This therapeutic approach involves directly introducing genetic material (usually DNA or RNA) into cells to alter genetic information and biological functions. Although it has shown promise in preclinical research and early-phase clinical trials, it still faces many challenges, including ensuring the safety of the treatment, improving the efficiency of gene delivery, and avoiding immune responses.
From May 2005 to March 2024, in total, there are at least 150 clinical studies related to oncology mRNA, including 89 clinical studies on mRNA drugs, 40 studies on mRNA therapies targeting dendritic cells (DCs), 18 studies on neoantigens, two studies on the treatment of HBV-positive HCC, and 1 study on the modulation of immune factors (Supplementary Table 2 ). Among them, the development of research targeting neoantigens has progressed rapidly since the onset of the COVID-19. Thirteen related clinical trials took place between 2022 and March 2024. Furthermore, 53.9% (48/89) of mRNA drug studies have seen an explosion in activity since the start of COVID-19. These results highlight the fervent pace of mRNA research. mRNA has shown promising efficacy in encoding neoantigens for cancer vaccines, tumor-associated antigens (TAAs) for activating dendritic cells, tumor suppressor factors for inhibiting tumor progression, CAR for engineered T-cell therapies, and genome-editing proteins for gene therapy. We have the details of mRNA vaccines in the following part.
mRNA therapy is an emerging medical technology that uses mRNA-based molecules to treat or prevent diseases. Compared to other therapies, mRNA technology has numerous advantages. It can produce a variety of vaccines and therapeutic drugs in a shorter time frame. The production cycle for mRNA, from In Vitro Transcription (IVT) to the preparation of mRNA-LNP complexes, takes approximately 10 days. 200 Upon administration into the organism, mRNA does not integrate into the host genome, therefore, the risk of mutations can be avoided. It degrades in the body after a brief period of action, resulting in no long-term toxicity. Moreover, mRNA therapy does not require a viral vector, which also reduces the risk of infection.
However, there are also some disadvantages of the mRNA treatment. First, delivering mRNA molecules to specific cells or tissues presents challenges and requires effective delivery systems, such as lipid nanoparticles (LNPs). Secondly, naked mRNA molecules are less stable in vitro and are susceptible to degradation by RNases, necessitating higher-quality storage and transportation systems. Thirdly, while mRNA therapies are generally considered to have low immunogenicity, in some cases, they may activate the immune system, leading to adverse reactions. Fourthly, targeting tissues other than the liver (which is the primary target for mRNA therapies) requires improved delivery systems. Additionally, the long-term effects and safety of mRNA therapies are not yet fully established.
ZFNs technology
Genome-editing modalities, including zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the CRISPR/Cas9 system, enable targeted genome modification in eukaryotic cells. They induce double-strand DNA breaks (DSBs) at specific loci and leverage cellular repair mechanisms, such as non-homologous end joining (NHEJ) or homology-directed repair (HDR), to precisely edit genetic sequences. 201 , 202 , 203 Moreover, the emergence of Prime Editing has expanded the scope of precise genetic manipulation. These methods have significantly advanced cancer gene identification, tumor cell epigenetic regulation, targeted delivery systems, the development of oncological animal models, and the clinical application of cancer immunotherapy and prophylaxis. 204 , 205 , 206
Zinc-finger nucleases (ZFNs) represent a sophisticated gene-editing platform that integrates the sequence-specific recognition of zinc finger proteins (ZFPs) with the DNA cleavage function of the FokI nuclease. This technology enables the precise targeting and cleavage of predetermined genomic sequences, thereby triggering the cell’s intrinsic repair machinery to execute site-specific genetic modifications (Fig. 6a ). 207 Predominantly, naturally occurring restriction enzymes recognize palindromic sequences of 4–6 base pairs (bp), whereas a solitary zinc-finger module, comprising approximately 30 amino acids, discerns a 3-bp DNA segment. 208 By arranging multiple zinc-finger modules in a tandem array, ZFNs can discern sequences ranging from 9 to 18 bp, thereby achieving remarkable specificity within the expansive human genomic landscape, which encompasses approximately 6.8 billion base pairs. 209 This advancement has been pivotal for the accurate localization of genetic sequences across the human genome. 210
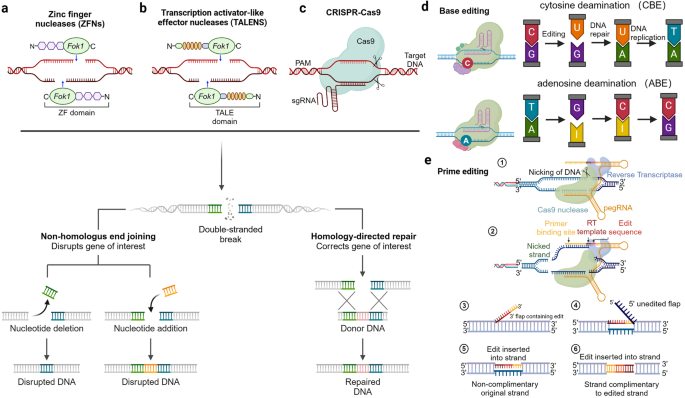
Various modes of genome editing. a ZFNs: By designing multiple zinc finger proteins to bind with specific DNA sequences, they guide the nuclease to cut the target gene, achieving precise gene editing through NHEJ and HDR. b TALENs: Specific transcription activator-like effector molecules are fused with nuclease domains. Two TALEN modules are necessary to bind to the target site, with a FokI nuclease fused to the DNA-binding domains, enabling precise gene editing through NHEJ and HDR. c CRISPR-Cas9: This system employs the Cas9 protein, guided by RNA molecules, to enable precise DNA cleavage at specific target sequences. It achieves accurate gene editing through NHEJ and HDR. d Base editing: an advanced form of Cas genome editing that enables the substitution of specific bases without inducing double-stranded breaks. This technique is categorized into two main types: C•G to T•A Base Editors (CBEs) and A•T to G•C Base Editors (ABEs). e Prime editing: The procedure involves constructing a prime editor complex, which includes a Cas domain, an RT (reverse transcriptase) domain, and a pegRNA. This complex searches for the DNA segment containing the target mutation and introduces a nick just in front of the mutation site. The nicked DNA strand then serves as a primer for DNA synthesis within the RT complex. The corrected, nicked strand is preferentially used over the original diseased strand, and the cell’s natural DNA repair mechanisms subsequently remove the diseased strand. This figure was created with Biorender.com
Furthermore, the strategic pairing of two ZFNs with distinct sequence specificities can facilitate cleavage at non-inverted repeat sites within a genome, provided they are correctly positioned and oriented. 209 In the realm of cancer therapeutics, ZFNs have been harnessed to modulate both tumor cells and T cells. A clinical trial has documented the efficacy of ZFN-603 and ZFN-758 in treating high-risk human papillomavirus (HPV)-associated cervical intraepithelial neoplasia (CIN). 211 , 212 Additionally, the employment of adeno-associated virus serotype 6 (AAV6) as a delivery vector has enabled the efficient correction of CCR5 or AAVS1 in CD8 and CD4 T cells. 213
Despite ZFNs’ potential in cancer therapy, they encounter several clinical challenges. The synthesis of ZF proteins is less modular than the CRISPR system, necessitating intricate protein engineering for the design and assembly of DNA recognition sequences, which is both technically demanding and costly. Moreover, ZFNs are susceptible to off-target effects and non-specific binding. To mitigate these issues, researchers have engineered single-residue substitutions within the FoKI domain, which maintain on-target activity while diminishing off-target efficiency, exemplified by a 98% reduction in TRAC gene expression in T cells. 201 However, ZFNs exhibit lower efficiency compared to other gene-editing modalities, such as CRISPR/Cas9. With the advent of CRISPR/Cas9, ZFNs have receded from the forefront of gene therapy for cancer. Nonetheless, ZFNs retain a significant role in specific applications where extreme sequence specificity is paramount, particularly in gene-editing tasks that require high precision.
TALEN technology
Followed the footstep of ZFNs technology, the TALEN technology was developed in 2010. 214 TALEN is composed of a non-specific DNA cleavage domain and a sequence-specific DNA-binding domain, which contains a highly conserved repeat sequence from transcription activator-like effector (TALE). Two TALEN modules are required to bind to the target site, and a FokI nuclease is fused to the DNA-binding domains to cleave DNA (Fig. 6b ). The DNA binding domain of TALENs is composed of reiterated TALE modules, wherein the 12th and 13th amino acids within each module dictate the precise DNA binding site. By strategically altering these amino acids, TALENs can be engineered to recognize virtually any DNA sequence with high specificity. Also, because the TALENs must bind to adjacent target sites simultaneously to elicit a DSB, this reduces the chance of off-target effects compared to other technologies.
In contrast to ZFNs, which utilize a 30-amino-acid motif to bind three base pairs of DNA, TALENs necessitate 33–35 amino acids to identify a single base pair. 215 This disparity in size can impede the co-delivery of both TALEN monomers within a single viral vector, particularly when packaging capacity is constrained. Furthermore, the propensity for instability in tandemly repeated sequences, as observed in TALENs, presents challenges for viral vector-based delivery. It is well known that multiple sequence repeats in TALEN genes hampers the use of lentiviral vectors. The lentiviral vectors can efficiently package TALEN-encoding mRNAs, but recombination during reverse transcription impedes successful delivery. 216 Adenoviral systems are recommended as a valuable TALENs gene delivery platform. 217
Although TALEN technology is not currently the prevalent method in the application of CAR-T therapy for cancer, it has been employed in specific research initiatives. A salient study from 2015 demonstrated the strategic use of TALENs to inactivate the TCR and CD52 genes within the CAR19-T cells, thereby averting the host’s immune rejection typically encountered in autologous CAR-T therapies and advancing the prospect of allogeneic CAR-T cell transplantation in CAR19 T treatment. 218 Additionally, there’s a clinical trial about designated TALENs (T27 and T512) targeting HPV16 E6 and E7 to induce cell apoptosis in CIN. 219 In summary, TALENs offer straightforward design and broad gene-targeting capabilities, but their potential in cancer therapy necessitates further exploration.
CRISPR therapy
Crispr/cas9 therapy.
Clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein (Cas)9 (CRISPR/Cas9) is a revolutionary gene-editing technology that allows researchers to make precise and efficient edits in the genome of cells. CRISPR-related gene-editing technology is presently regarded as one of the most prominent tools. The most used CRISPR system is the Type II CRISPR-Cas9 system from Streptococcus pyogenes (SpCas9). It involves the utilization of the Cas9 protein, which is directed by RNA molecules, to facilitate precise DNA cleavage at targeted sequences. After the DNA cleavage, the cell’s inherent repair pathways can be harnessed to either incorporate or excise genetic material (Fig. 6c ). This CRISPR-based therapeutic intervention holds promise in the treatment of genetic disorders through the rectification of pathogenic mutations and has additionally been investigated as a prospective therapeutic strategy for malignancies.
CRISPR has become a highly refined technology for gene editing in ex vivo adoptive T-cell therapies. Numerous clinical studies have employed CRISPR to eliminate PD1 expression on T cells to enhance their anti-cancer properties. 220 , 221 , 222 , 223 , 224 , 225 But since this technology is still very new, only one clinical trial (NCT02793856)—a dose-escalation study of ex vivo knocked-out, expanded, and selected PD-1 knockout T cells from autologous origin—has been completed. Others are ongoing. One ongoing clinical trial (NCT03545815) evaluates the feasibility and safety of CRISPR-Cas9 mediated PD-1 and TCR gene-knocked out chimeric antigen receptor (CAR) T cells in phase 1 patients with mesothelin-positive multiple solid tumors. Another ongoing trial (NCT03081715) plans for 16 advanced esophageal cancer patients to receive two cycles of CRISPR knockout PD-1 engineered T cell infusion. Knocking out HLA in allogeneic T cells to reduce immune rejection is also a common procedural approach. 226 A clinical study initiated on March 20, 2024 (NCT06321289), employs the CRISPR-Cas9 gene-editing tool to simultaneously knock out the endogenous receptor α constant (TRAC), human leukocyte antigen (HLA)-A/B, CIITA, and programmed death 1 (PD-1) genes in T cells from healthy donors to observe the reduction in GvHD toxicity with this strategy. Additionally, CRISPR-Cas9 serves as a mainstream technology for synthetic lethal screening of drugs, aiding in the development of anticancer drugs. Current exploration areas mainly include intrinsic cellular mechanisms, such as interactions between BRCA-PARP 227 and BRAF-EGFR. 228
However, applying CRISPR directly in the body raises several key issues, including precise targeting to avoid unintended effects, preventing unnecessary immune reactions, and ensuring the long-term efficacy and stability of the treatment. It is crucial to select an appropriate in vivo delivery system. Clinical trials using AAVs as a delivery system for CRISPR in vivo cancer treatment in patients have not yet been conducted. However, preclinical studies have shown a wealth of potential applications in brain tumors, 229 , 230 lung cancer, 231 , 232 , 233 , and liver cancer. 234 , 235 , 236
Base editing
Base editing is a modified Cas genome editing method that can replace specific bases without any double-stranded breaks. It is split into two classes: C•G to T•A Base Editors (CBEs) and A•T to G•C Base Editors 237 (ABEs). CBEs consist of 3 components: Cas9n for DNA locus binding, a cytidine deaminase enzyme to transform cytosine to uracil, and an Uracil Glycosylase Inhibitor (UGI) to protect the uracil intermediate from Uracil DNA Glycosylase 237 , 238 (UDG). ABEs consist of Cas9n and an adenosine deaminase. 237 , 239 The Cas9n first binds to the target locus, allowing the cytidine deaminase or adenosine deaminase to deaminate C or A respectively, nicking the top strand, and allowing the cell’s indigenous DNA repair systems tothe DNA to repair the damage and edit the DNA to the correct base 237 , 239 (Fig. 6d ).
The fusion of rat APOBEC1 (rAPOBEC1) and Cas9 constitutes the earliest version of the cytosine base editor (BE1). Subsequently, by introducing UGI and modifying the activity of Cas9, researchers have developed more efficient versions of CBE, such as BE2, BE3, and BE4. Improved Cas9 variants such as eCas9, Cas9-HF are used to decrease off-target base editing 237 , 240 ; BE3-Gam and BE4-Gam, where the Gam protein protects DSBs in DNA, are used to reduce accidental locus-based indels and increase product purity, and many other variants are produced. 241
Base editing can be used in a cancer therapeutic setting by improving CAR-T technology or direct base editing. Studies have shown that autologous CAR-T cells may be produced through multiplex gene KO using base editors when combined with cell sorting. A current limitation of CAR-T therapy is the cost of allogenic CAR-T cells being used. The use of autologous CAR-T cells would require KO of MHC I and II, which can be done much more efficiently with BE combined with cell sorting. 242 Alternatively, one study by Sayed et al. shows the potential of modifying genetic mutations in cancer cells using base editing by correcting KRAS and TP53 mutations on cancer organoids. 243
Prime editing
Prime editing is a new modified Cas genome editing method that is able to search and replace the targeted gene without the need for double-strand breaks or donor DNA. 244 The procedure works by constructing a Prime Editor complex (PE) with a Cas nickase domain and Reverse Transcriptase (RT) domain along with a pegRNA, which will have its search domain and replace domain on the same strand, 244 with a spacer on the 5’ end and the primer binding site and edit template on the 3’ end. The Prime Editor complex searches for the DNA with the target mutation and nicks the strand just in front of the mutation. 244 The nicked DNA strand then primes for DNA synthesis in the RT complex. 244 The corrected nicked strand is preferentially preferred over the diseased original strand, and the cell’s indigenous DNA repair mechanisms will excise the diseased strand. 244 The PE will search for the protospacer adjacent motif (PAM) and align the photospacer with the spacer on the pegRNA. 244 The PAM strand will be nicked and the 3’ side of the pegRNA will hybridize with the PAM strand, allowing the RT to polymerize edited DNA on the target site. 244 The PE will then leave the strand, leaving the edited DNA with a 3’ flap containing the edit. 244 The 3’ flap replace the original unedited strand, leaving the unedited 5’ flap to be removed. 244 The mismatched DNA strand is then repaired to incorporate the edit 244 (Fig. 6e ).
The advantages of prime editing are multifold. It is capable of executing all different types of single-base substitutions, multiple-base substitutions, and small indels. 245 It also has high editing and on-target precision, above contemporary CRISPR-Cas9 and other base editing methods. 245 However, prime editing also involves many complicated steps, and despite having fewer moving parts than the other methods. Hence, many of the first developmental efforts involved improvements to the PE system. The first version of PE, hereby called PE1, have a low efficiency, and subsequent versions developed incorporated improved RT domains (PE2), 244 optimized pegRNAs(PE3, G-PE, sPE, ePE), 244 , 246 improved nicking strategy (PE3, PE3b), 244 the addition of transient expression of a dominant MMR protein (MLH1dn) (PE4,PE5), 247 addition of the sgRNA for nicking, enhanced nuclear localization (PE*, PEmax), 246 , 247 and many other modifications. A possible drawback of the PE system may include genotoxicity to hematopoietic stem cells, according to Fiumara et al., that while the PE system have a higher accuracy that Cas9 and Base Editing, PE can still induce DNA DSBs and deletions at the target site. 248
Prime editing is a versatile tool with many potential applications in cancer modelling and therapy. A study by Liu et al. has demonstrated the use of prime editing in cancer modeling by increasing tumor burden through somatic engineering, mutating the β-catenin gene with PE2* to increase tumor formation rates. 249 A recent study by Davis et al. has confirmed that prime editing can be delivered and successfully executed in the mouse brain, liver, and heart using dual AAV delivery, suggesting a possible therapy for hard-to-reach areas; 250 meanwhile, a study by Abuhamad et al. is able to revert TP53 mutation using prime editing breast cancer cells. 251 A study by Jang et al. has similarly been able to correct oncogenic G12 and G13 KRAS mutations with prime editors in HEK293T/17 and human cancer cells in vitro. 252 Currently, Prime Medicine, a start-up created by the original inventors of prime editing A. Anzalone and D. Li, had made some headway by multiplexing Prime Editing with PASSIGE, creating CAR-T cells by non-viral one-step delivery with high efficiency. 253
Delivery system
Currently, adeno-associated viruses (AAV) and lipid nanoparticles (LNP) are among the most advanced delivery systems in clinical use.
AAV delivery system
AAV, a single-stranded DNA virus without an envelope, has a genome length of approximately 4.7 kilobases (kb) and features two T-shaped inverted terminal repeats (ITRs). The genome encodes multiple proteins, including Rep and Cap proteins, which play roles in viral replication, assembly, and packaging. Different AAV subtypes have different tissue specificity. Selecting appropriate AAVs can achieve efficient gene delivery in specific tissues, thereby providing more precise and effective means for gene therapy. For example, the AAV9 subtype has a higher affinity for the heart and liver, while the AAV2 subtype exhibits greater selectivity for retinal cells. 254 AAVs are widely present in humans and animals and is generally considered non-pathogenic. It has become the preferred delivery vector for gene therapy due to its broad host range, low immunogenicity, low cytotoxicity, multiple serotypes, and low frequency of integration into the host genome. However, there’s some limitations of the AAV delivery system. First of all, AAV’s limited packaging capacity and gene length restriction restrict its ability to carry payloads larger than 5 kb such as the SpCas9 protein. To overcome this problem, smaller CRISPR constructs, such as SaCas9, Nme2Cas9, Cas12f, Cas12j, sgRNA, etc., were used. 255 Also, the approach to split the payload into two separate constructs allowed for more efficient packaging and delivery using viral vectors such as AAVs. Another strategy involves the use of overlapping AAV vectors, which can enhance the recombination efficiency and encourage natural homologous recombination (HR) between the vectors. Additionally, trans-splicing vectors are utilized to achieve precise and controlled gene expression. These vectors contain a splicing donor sequence that can be spliced into the target gene, leading to the production of a functional protein. Split inteins, on the other hand, are used to facilitate the splicing of two protein fragments, allowing for the reconstitution of a functional protein. Another concern with AAVs treatment is its potential impact on the liver. Reports indicate that AAV vectors have a high affinity for the liver. 256 When targeting the liver, a lower dose is required, whereas higher doses are needed to treat other tissues. However, clinical trials with higher doses of AAVs have raised the risk of liver-related side effects. Several children died from liver function abnormalities after receiving high doses of AT132 (3×10^14 vg/kg in 2020; 1.3×10^14 vg/kg in 2021). 257 In 2022, Novartis reported that two children died from acute liver failure after treatment with Zolgensma. 258 Five Airedale terriers with hemophilia A developed cellular proliferation in the liver after receiving AAV8 delivery of the FVII gene. 259 These issues have sparked controversy surrounding AAV therapy. Reducing the dosage of AAVs is a reliable measure to lower the risk. Sharif Tabebordbar et al. found that MyoAAV can be used for treatment with smaller doses, especially MyoAAV2A-CK8 for mouse treatment, which is 100 times lower than the dose used in the AT132 study. 260 ASC Therapeutics has enhanced the secretion efficiency of therapeutic proteins by special gene modifications and optimization of the cellular unfolded protein response (UPR), thereby reducing the required dose of AAV in gene therapy. 261 These approaches offer innovative ways to optimize the delivery and expression of therapeutic genes, providing researchers with tools to fine-tune gene expression and enhance the efficacy of gene therapies.
LNP delivery system
Lipid nanoparticle (LNP) systems have shown promise in the delivery of CRISPER therapeutics. These systems are composed of four main lipid components: ionizable lipids, helper lipids, cholesterol, and polyethylene glycol (PEG)-ylated lipids. These components form the structure that encapsulates and protects mRNA molecules, enabling them to resist degradation in the body and effectively deliver to target cells through the bloodstream. Compared to viral vectors, LNPs have numerous advantages. First, they have strong design flexibility because they are composed of four lipids, and the ratio of different lipids can be adjusted to achieve various effects. Additionally, they can anchor other ligands onto their own carriers. For example, tumor-specific antigens (TSAs) or hydrophobic antibody derivatives can be anchored on the surface of functionalized LNPs to enhance their targeting capabilities. 262 Second, as a non-viral carrier, LNP generally has better safety and lower immunogenicity compared to viruses. Moreover, LNPs possess an innate adjuvant effect, which can promote CD4 + T helper 1 (TH1) mediated anti-cancer responses. 263
For its disadvantage, the current side effects of LNPs mainly fall into three categories: allergic reactions, immune responses, and liver effects. At the same time, due to capacity limitations, LNPs have certain constraints when delivering complex genes and base editing. At present, the issue of liver burden can be addressed by optimizing the ionizable lipid and reducing the dosage. The percentage of PEG-lipids, cholesterol, and helper lipids, such as 1,2-distearoyl-sn-glycero-3-phosphocholine, can be adjusted to enhance its stability and reduce toxicity. 264
LNP, as a delivery system for mRNA vaccines, has been successfully used for prophylactic vaccines against pathogens and is being tested in clinical trials in oncology The targeting efficiency of different LNPs in combination with sgRNA within various tissues in patients needs additional research. One preclinical data has demonstrated that targeted sgPLK1-cLNPs significantly enhance survival rates, achieving an 80% improvement in ovarian tumor mice models. 265 The other two target PLK1 in A375 tumor-bearing mice 266 and HepG2 tumor bearing mice 267 didn’t show sufficient response. The application of LNPs for the delivery of CRISPR in cancer therapy is still in its early stages and requires further research and clinical trials to validate its safety and efficacy.
PNPs delivery system
In addition to lipid nanoparticles, polymer nanoparticles (PNPs) are another promising delivery system for gene therapeutics. PNPs are widely used as drug delivery systems, offering controlled release of drugs, enhancing the solubility of poorly soluble drugs, and targeted delivery to tissues or cells. A critical advantage of PNPs is similar to LNPs for customization and integration of diverse physicochemical characteristics to enhance their effectiveness. Moreover, PNPs are easy to be engineered as carriers, creating drug delivery systems that are both non-toxic and biocompatible. Several studies have been conducted on the use of PNPs as carriers for CRISPR in cancer applications. One study reported that they successfully established a co-delivery PNPs system composed of sg-VEGFR2 and Cas9, which led to more than 70% suppression of HepG2-induced HCC tumor progression. 268 Additionally, Wu et al. utilized an sgHMGA2 PNPs system to achieve high-efficiency delivery (95%) and editing (82%) in gastric cancer. 269 This system was shown to decrease immune responses in mice that actively targeted breast cancer tumor cells, aiding in the prevention of tumor re-growth upon re-challenge. Moreover, this approach was found to induce immunological memory, conferring resistance to lung metastatic spread. 270 As the application of PNPs in gene editing is still in its infancy, no adverse events have been reported to date.
Challenges to gene editors
In the development of gene therapies, simplicity of design is important, but it must be balanced with the complexity of achieving tropism and avoiding off-target effects. Long-term nuclease expression requires longer safety studies due to mutations, and in vivo characterization is necessary to account for species differences between NHPs, mice, and humans. 263 Delivering therapies to organs other than the liver requires further study, and the limitations of different delivery systems must also be considered.
Neoantigen and cancer vaccine
Genomic instability and mutations are fundamental characteristics of tumorigenesis, enabling the hallmark capabilities of cancer, including the maintenance of proliferative signaling and resistance to cell death. 271 Concurrently, mutations can generate aberrant peptides that are presented by the major histocompatibility complex (MHC), which may activate anti-tumor adaptive immunity by lymphocytes. 272 , 273 This process underlies the principle of neoantigens and cancer vaccines. Given the unique expression of neoantigens in tumor cells, they are regarded as an ideal target for tumor vaccines.
Neoantigen identification and validation
Genetic alterations in cancer may result from single nucleotide variants, insertions, deletions, gene fusions, and oncogenic virus infections. 274 , 275 , 276 , 277 Thanks to the development of multi-omics technologies, including genome sequencing and mass spectrometry for the proteome, it is now possible to efficiently screen for potential neoantigens in a large pool. Through next-generation sequencing data of tumor genomes or transcriptomes compared to normal ones, neoantigens can be preliminarily predicted in silico. 278 Subsequently, computational algorithms that analyze the binding affinity between MHC molecules and the predicted neoantigens/peptides are used to select optimal candidates. 279 , 280 Following bioinformatic prediction, experimental validation should be conducted to assess the TCR recognition of the peptide-MHC (pMHC) complexes using T cells derived from patients or healthy donors 281 , 282 (Fig. 7 ). However, reports indicate that only a few bioinformatically predicted neoantigens/peptides are recognized by T cells, 281 leading to controversy regarding the reliability of these predictions. An alternative approach to obtain immunogenic neoantigens/peptides involves loading autologous antigen-presenting cells (APCs), such as dendritic cells (DCs), with tumor lysates and validating them ex vivo by co-culturing the lysate-pulsed DCs with T cells. 283
The process of cancer vaccine development . The preparation of cancer vaccines is a meticulous process involving multiple steps, aimed at developing vaccines that can stimulate the immune system to target cancer cells. This process begins with the selection and identification of specific tumor antigens, followed by the design of the vaccine, which may include peptides, recombinant proteins, genetic vectors, or dendritic cells. Subsequently, the vaccine undergoes immunological testing in vitro and is evaluated for its safety and efficacy in animal models. After successfully passing preclinical research, the vaccine proceeds to human clinical trials, including Phase I, II, and III trials, to assess its safety, immunogenicity, and therapeutic effectiveness. This figure was created with Biorender.com
Vaccination
After screening and validation, immunogenic neoantigens can be used in various forms of vaccines, including peptides, nucleic acid-based vaccines, and lysate-pulsed dendritic cells (DCs).
Peptide vaccines
Peptides are the most common form of cancer vaccines, consisting of either a single synthetic peptide or a mixture of several synthetic peptides. 284 , 285 , 286 Clinical trials have confirmed that personalized peptide-based vaccines can induce tumor-specific immunity and long-term immune memory across various cancer types. 287 , 288 , 289 To enhance the immune response, immunoadjuvants are often used in conjunction with peptide-based vaccines, 290 , 291 as will be discussed in detail in the relevant section.
mRNA vaccines
mRNA vaccines have proven to be an efficient and safe therapeutic method during the COVID-19 pandemic, indicating a promising future in cancer immunotherapy. Compared to peptide-based vaccines, mRNA vaccines can encode full-length neoantigens or carry multiple neoantigens on a single mRNA molecule. 292 To ensure stability and intracellular delivery, nanoparticles, such as lipids, are often used to facilitate the transport of mRNA vaccines. 293 Several mRNA cancer vaccines have recently completed Phase I/II trials. Autogene cevumeran, an mRNA vaccine containing 20 personalized neoantigens delivered by lipoplex nanoparticles, showed T-cell expansion in 8 out of 16 pancreatic cancer patients in a Phase I study, with benefits in recurrence-free survival (RFS) after vaccination. 294 Another personalized mRNA vaccine, mRNA-4650, contains 20 neoantigens based on sequencing data from individual patients. A Phase I/II study involving four patients with metastatic gastrointestinal cancer who received mRNA-4650 vaccination showed no objective clinical responses, although the vaccine elicited T-cell responses against predicted neoepitopes. 295 Therefore, enhancing the immunogenicity, stability, and delivery methods of mRNA vaccines should be considered.
DNA vaccines
Similar to mRNA-based cancer vaccines, DNA vaccines are constructed using an expression vector, such as a plasmid, to encode the neoantigens identified through sequencing, 296 which are more stable and easier to store and transport. Due to these advantages, DNA vaccines are increasingly applied in clinical practice. In 2014, a Phase I study focusing on a plasmid-based mammaglobin-A (MAM-A) DNA vaccine reported that the vaccine was efficient in eliciting cytotoxic antigen-specific T cells, and vaccinated breast cancer patients experienced prolonged PFS compared to those who did not receive vaccination due to HLA-screening failure. 297 Another plasmid-based DNA vaccine targeting insulin-like growth factor binding protein-2 (IGFBP-2) also presented an activation effect on the T helper-1 immune response in ovarian cancer patients, as both IFNγ-secreting T cells and T-bet positive T cells were increased after immunization. 298 A double-blind, placebo-controlled clinical trial targeting human papillomavirus (HPV) proteins in patients with cervical intraepithelial neoplasia (CIN) provided more compelling evidence of the therapeutic effects of DNA vaccines. 299 Furthermore, one Phase IIb study aimed to assess VGX-3100, a double plasmid vaccine encoding HPV protein E6 and E7. After 36 weeks of follow-up, the result showed a significant difference in histopathological regression between the two groups (49.5% of the VGX-3100 group (107 patients) and 30.6% (36 patients) of the placebo group), indicating the potential of VGX-3100 as a non-surgical treatment option for patients with CIN2/3.
Dendritic vaccines
Since the dendritic cell is the key antigen-presenting cell to stimulate adaptive immunity, 300 neoantigen-loaded dendritic cells (DCs) provide a direct way to initiate anti-tumor immunity. 301 Whole-tumor lysate is the most used method for neoantigen loading. DCVax-L, an autologous whole-tumor lysate-loaded dendritic cell vaccine, demonstrated improved survival both in patients with newly diagnosed and recurrent glioblastoma. 302 Additionally, synthetic peptides, based on sequencing and prediction analysis, can be used for antigen loading. A pilot study explored the efficacy of the personalized peptide-loaded dendritic cell vaccine, Neo-DCVac, in patients with advanced lung cancer. 283 The T-cell response assay verified a strong increase in IFNγ secretion and cytotoxicity, thereby strengthening the efficiency of tumor therapy.
The advantages, disadvantages, and challenges of cancer vaccine
Unlike other types of drugs, cancer vaccines are preventive in nature. Therefore, their first advantage is that they can effectively suppress the occurrence of tumors. In addition, research has shown that cancer vaccines have the potential to stimulate long-term immune memory, providing patients with lasting protection and reducing the risk of cancer recurrence. However, the main disadvantage of cancer vaccines is based on the issue of neoantigens. Due to the individual variability of antigens and the interference of the immune microenvironment, the efficacy of cancer vaccines targeting neoantigens exhibits inconsistencies.
Though great success has been achieved, it remains a pressing issue to be addressed in cancer vaccine. The most worrisome problem is the immunogenicity of the vaccine, as a considerable part of patients still showed no response to various cancer vaccines. One practical method is to add adjuvant along with vaccines to enhance innate immune response. Improving the form or sequence of synthetic peptides to enhance their efficiency of binding to MHC is another solution that could be taken into consideration.
Oncolytic virus
The history of oncolytic viruses (OVs) can be traced back to the early 20th century. An Italian woman suffering from cervical cancer was bitten by a dog after being injected with a rabies virus vaccine. Miraculously, the tumor disappeared, and the woman enjoyed a cancer-free survival for 8 years. 303 Though the underlying mechanisms of this phenomenon were uncertain at that time, physicians began to use rabies virus vaccines to treat patients with cervical cancer. A hundred years have passed, and OVs therapy has come into a new era, achieving significant success in treating a broad range of tumor types.
Oncolytic mechanisms
Oncolytic viruses can kill tumor cells through several direct and indirect mechanisms according to the design concepts. OVs tend to recognize surface markers that are abnormally overexpressed on cancer cells and then enter those targeted cells. The recognition pattern depends on the virus types and modification strategies. CD46 is often upregulated on tumor cells from leukemia, gastrointestinal cancers, gynecological cancers, and lung cancer, 304 which can be recognized by measles viruses and some subtypes of adenoviruses. 305 The most commonly used vector, herpes simplex viruses, utilize the herpesvirus entry mediator (HVEM) and nectin-1 to enter tumor cells. 306 , 307
Normal cells will induce the interferon pathway to limit viral replication after infection, while tumor cells are often deficient in this defensive mechanism. 308 , 309 Therefore, after entering tumor cells, OVs begin to rapidly replicate, finally leading to tumor cell lysis and releasing new viruses to infect surrounding tumor cells. 310 Accompanying cell lysis, damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), along with tumor antigens, are released into the tumor microenvironment (TME) and are subsequently sensed by antigen-presenting cells (APCs), including dendritic cells (DCs) and macrophages. 311 , 312 , 313 Once sensing these signals, APCs will stimulate T cells to induce adaptive immunity and tumor-specific cytotoxicity, thus triggering a systematic anti-tumor immune response. 314
Besides the common mechanisms, OVs can induce oncolytic effects through different modification methods, which will be discussed in the following parts.
Clinic application
Despite the long history of oncolytic viruses (OVs), most of the products are in clinical trial stages. Studies on OVs targeting other malignancies, including multiple myeloma, pancreatic cancer, lung cancer, and pediatric cancers, are ongoing, with details provided in Table 5 .
Talimogene laherparepvec (T-VEC) is the first US FDA-approved OV for patients with recurrent unresectable melanoma, improving the median overall survival to 23.3 months. 315 It is derived from herpes simplex virus type 1 (HSV-1) and features an insertion of the human granulocyte macrophage colony-stimulating factor (GM-CSF) gene. This insertion enables local expression of GM-CSF at the tumor site, recruiting antigen-presenting cells (APCs) such as dendritic cells (DCs) and inducing downstream T-cell activation. 316 In addition to melanoma, T-VEC’s effects on breast cancer, sarcoma, and head and neck squamous cell carcinoma are being evaluated in clinical trials, with initial results reported. 317 , 318 , 319
DNX-2401 is an adenovirus-based OV with a deletion in the E1A gene and an insertion of the RGD sequence, which confers glioma cell selectivity via integrins αvβ3 and αvβ5. 320 A phase I study confirmed the efficacy of DNX-2401 in patients with recurrent malignant glioma, noting increased CD8 T-cell infiltration in tumors. Among the participants, 3 out of 20 achieved complete responses and experienced a progress-free survival (PFS) of over 3 years. 321 Additionally, a recent study focusing on the combination of DNX-2401 with an immune checkpoint inhibitor has shown initial results indicating a survival benefit in select patients. 322
Another OV targeting glioma, G47Δ, is a third-generation engineered HSV-1. It is constructed by deleting the α47 gene and overlapping the US11 promoter from its parental vector, thereby enhancing anti-tumor immunity and reducing replication in normal cells. 323 Recent phase I/II studies have shown that G47Δ demonstrates good safety and provides survival benefits for patients with advanced glioma. 20 , 324
The advantage, disadvantage, and challenges for OVs
OVs offer numerous advantages. Firstly, they can selectively replicate within tumor cells while also activating the immune system, enabling them not only to directly kill cancer cells but also to serve as vectors for delivering therapeutic genes. Secondly, due to their inherent defects, tumor cells are more susceptible to viral infections, while normal cells are relatively safe as they possess mechanisms to clear viruses. Moreover, the small size of viral genomes and mature genetic engineering techniques make the efficient and cost-effective modification of oncolytic viruses possible, facilitating the specific targeting of cancer cells. Lastly, the multifaceted oncolytic effects of these viruses, include increasing tumor antigen exposure, modulating the tumor microenvironment, and activating the immune system. As the development of ICIs in tumor therapy, the combination of OVs with ICIs is gaining more attention. 325 , 326 , 327 , 328 It is practical to find molecular markers to predict the population who would benefit from combination therapy through multi-omics technology.
When it comes to the drawbacks of oncolytic virus therapy, there are several key issues. First, there is the issue of targeting specificity. Although oncolytic viruses are selective, their specificity in targeting tumors needs improvement to ensure effective infection of the intended cancer cells. Second, there is the issue of immune response. Patients may develop an immune reaction to the oncolytic viruses, potentially limiting the treatment’s efficacy and the possibility of its repeated use. Third, determining the appropriate viral dosage for treatment is challenging. Additionally, controlling the effective propagation of the virus within target cells presents difficulties.
Though OVs have shown efficacy in a wide range of malignancies, there remains many challenges to be faced. One is the treatment-related adverse events including some severe reactions such as lymphopenia, and thrombocytopenia, 20 , 329 , and combination with other therapy may increase the risk. 330 It is currently uncertain how OVs caused those adverse events, perhaps related to virus load and tumor necrosis. 331 Several measures have been taken to reduce the virulence and risks of adverse events. The most utilized method is insertion or deletion of some specific genes in OVs to limit its replication and infection to normal tissues. For example, retinoblastoma, a common pediatric cancer, often results from retinoblastoma 1 gene (RB1) dysfunction, causing an increase in the abundance of E2F. 332 , 333 According to this genetic change, VCN-01 was constructed by inserting a E2F-responsive element, E2F1 promoter, to enhance the expression of E1A in E2F-sufficient cells while low replication capability in normal cells. 334 Another gene-modified OVs, JX-594, was deficient in thymidine kinase (TK) gene and showed a selectivity to tumors with elevated cellular TK abundance. 335 Thus, genetic modifications to control the expression of virulence-related or add a switch for replication will be the solution to adverse events.
Another issue that needs to be improved is the administration or delivery way of OVs, since most of the OVs are administrated through intratumoral or intracavitary injection to achieve local concentration, which is easy to perform in some superficial or abdominal cancers. 317 , 336 , 337 However, malignancies such as glioma are hard to reach due to their anatomical location, which sets obstacles for OVs administration. To address this issue, some new delivery systems were applied. Adenovirus-based CRAd-S-pk7 was loaded by neural stem cell line to enhance its ability to target glioma and migration across the blood-brain barrier, though the injection of viruses was taken after surgical resection. 338 Another study loaded the virus, Icovir-5, with autologous, bone marrow-derived mesenchymal stem cells (MSCs) and administrated it through intravenous injection upon the tumor-homing effect of MSCs. 339 From this perspective, human-derived cells with specific tissue tropism could act as an ideal carrier to take OVs to tumor site.
Immunologic adjuvant and innate immunity activator
Although current cancer immunotherapies primarily focus on enhancing the adaptive immune response, such as immune checkpoint inhibitors to promote T-cell cytotoxicity, 340 methods aimed at activating the innate immunity within tumors are gaining more attention. In 1890, the American surgeon William Coley accidentally discovered that a patient with both sarcoma and erysipelas experienced spontaneous tumor regression. This observation encouraged him to use the mixed toxins from Streptococcus erysipelas and Bacillus prodigiosus, known as Coley’s toxins, to treat patients with sarcoma. 341 Although some patients experienced regression after receiving Coley’s toxins, skepticism about Coley and his toxins has always persisted. 342 Now, it has become clear that the bacterial components may activate the innate immunity and induce an anti-tumor effect. 204 Therefore, therapies such as Coley’s toxins and immunologic adjuvants, which aim to stimulate the innate immune system, could represent a novel approach to treating cancer. 343
Bacillus Calmette-Guérin (BCG)
Despite the fact that intravesical administration of BCG in bladder cancer patients has long been the standard care, backed by solid evidence from clinical practice, 344 the underlying mechanisms are not yet fully understood. BCG administration can induce the secretion of proinflammatory cytokines from tumor cells and promote immune cell infiltration. 345 It is reported that NK cells can be activated by BCG, potentially playing a significant role in the tumoricidal process. 346 In addition to stimulating the innate immune response, BCG can also elicit an adaptive immune response against bladder cancer. This may be a consequence of increased antigen-presenting efficiency following the activation of innate immune cells. 347 In summary, further efforts should be dedicated to elucidating the immunotherapeutic effects of BCG, which could provide valuable insights for developing novel treatment strategies for other cancer types.
Toll-like receptors (TLRs) agonists
The family of Toll-like receptors (TLRs) comprises several members that are key regulators of innate immunity and initially garnered attention for their role in infections. 348 The activation of various TLRs in antigen-presenting cells, such as macrophages and dendritic cells, can induce the secretion of proinflammatory cytokines and subsequently stimulate adaptive immunity in T cells. 349
The TLR3 agonist, poly-ICLC, is frequently used as an adjuvant with cancer vaccines and has demonstrated good safety and efficacy. 350 , 351 Similarly, lipopolysaccharide, a major component of Gram-negative bacteria, is the natural ligand for TLR4 and can also be employed as an adjuvant in cancer vaccines. 352
In addition to being combined with cancer vaccines, TLR agonists can be administered as monotherapy or neoadjuvant therapy for certain malignancies. A phase I study investigated the efficacy and safety of G100, a synthetic TLR4 agonist, in patients with Merkel Cell Carcinoma. 353 Following intratumoral injection, G100 significantly increased the infiltration of T cells, activated the immune response, and led to sustained tumor regression in patients. Motolimod, a TLR8 agonist, when combined with chemotherapy and cetuximab, achieved improved outcomes compared to placebo in patients with HPV-positive head and neck squamous cell carcinoma. 354
The advantage, disadvantage and challenge of Immunologic adjuvant and innate immunity activator
Adjuvants and innate immunity activators serve as essential tools in enhancing vaccine efficacy and activating the immune system. Adjuvants enhance the protective power of vaccines by strengthening immune responses in a non-specific manner. They reduce the necessary amount of antigen and meet the immune needs of diverse populations. However, their use is accompanied by safety concerns, including local or systemic adverse reactions, as well as concerns about long-term safety and potential side effects. Innate immunity activators rapidly respond to pathogen infections, particularly promising in antiviral and cancer treatments. Yet, these activators may exhibit a lack of specificity, sometimes leading to excessive inflammatory responses, and resistance issues may arise with long-term use. Consequently, despite the significant potential of adjuvants and innate immunity activators in the medical field, careful consideration is necessary in their development and application to ensure safety and efficacy.
While we have gained some understanding of immunologic adjuvants and innate immunity activators, the complex interactions between innate and adaptive immunity within the tumor microenvironment have not yet been fully deciphered. The challenge of fully elucidating the mechanisms of Coley’s toxins through multidisciplinary approaches remains significant.
Proton therapy and carbon ion therapy
Proton therapy and carbon ion therapy are types of charged particle radiotherapy characterized by the Bragg peak, which offers advantages over conventional photon radiotherapy. 355 This means that proton and carbon ion therapy deliver a lower radiation dose to surrounding tissues and reduce adverse effects. 356 , 357 In 1990, the first hospital dedicated to proton therapy opened in Loma Linda, 358 and the technology has since matured for cancer treatment. Due to higher costs and the technical demands of heavy ion accelerators, carbon ion therapy is less commonly applied in clinical practice, despite its potential advantages over proton therapy. 359
Head and neck cancer
Radiation therapy is an indispensable treatment modality for head and neck cancer. While proton therapy has been shown to have lower toxicity to surrounding tissues compared to photon therapy, 360 its efficacy superiority remains controversial. 361 A more recent retrospective study evaluated the survival benefits of proton therapy in patients with recurrent head and neck squamous cell carcinoma. 362 Those who underwent salvage surgery followed by fractionated proton therapy experienced significantly improved local control rates and survival compared to patients who received salvage surgery with photon re-irradiation in previous studies. Two independent centers reported that carbon ion therapy demonstrated certain efficacy and safety in treating locally advanced head and neck cancer, particularly in cases of malignant melanoma and adenoid cystic carcinoma. 363 , 364

Non-small cell lung cancer (NSCLC)
Passive scattering proton therapy (PSPT) has shown advantages in safety, with a decreased rate of adverse events in unresectable NSCLC compared to historical data from patients receiving photon radiation therapy. 365 A comparative study involving locally advanced NSCLC indicated that intensity-modulated proton therapy (IMPT) delivered lower radiation doses to surrounding organs, including the heart, lung, and esophagus, and resulted in a lower rate of adverse events compared to PSPT, although no significant difference in survival was observed. 366 However, a recent study comparing the toxicity of intensity-modulated photon radiotherapy (IMRT) with PSPT concluded that there was no difference in radiation pneumonitis or local failure observed between the two groups. 367 Therefore, further explorations and adjustments are necessary to determine the efficacy and safety of proton therapy.
Regarding carbon ion therapy, two dose escalation studies conducted by the National Institute of Radiological Sciences in Japan have investigated its efficacy and safety in patients with stage I NSCLC. 368 , 369 These preliminary results suggest that single-fraction carbon ion therapy is effective and safe, even in elderly patients, although further studies for various stages of patients are needed.
Prostate cancer
Due to the anatomic position of the prostate, radiation-related gastrointestinal and urinary injuries, erectile dysfunction, and hip fractures are distressing adverse events that cause secondary harm to patients. Previous research has shown that the benefits of proton therapy compared to photon radiation in prostate patients are controversial. A retrospective study compared toxicity and disease control rates in nonmetastatic prostate cancer and found that the proton therapy group had a higher rate of gastrointestinal morbidity than the IMRT group, although no difference in the rate of additional therapies between the two groups was observed. 370 Another prospective study compared target coverage and doses to organs at risk between proton therapy and IMRT in patients with low-intermediate prostate cancer, also yielding a negative result. 371 Data on carbon ion therapy for prostate cancer is limited, as only research groups from Japan and Germany have conducted large-scale cohort studies. According to German data, carbon ion therapy showed lower effectiveness and worse survival compared to proton therapy in patients with primary prostate cancer. The five-year overall and progression-free survival rates in the carbon ion group were 91% and 50%, respectively, compared to 98% and 85% in the proton therapy group. 372 However, data from Japan demonstrated better efficacy of carbon ion therapy, with five-year overall survival rates of 100%, 99%, and 96% for low-risk, intermediate-risk, and high-risk patients, respectively, and biochemical recurrence-free survival rates of 92%, 89%, and 92%, respectively. 373 Given the sample size, the results from the Japanese cohort ( n = 2157) were more convincing than those from the German cohort (n = 92). However, further research is needed to verify the efficacy and safety of carbon ion therapy in different countries and among diverse populations.
The advantages, disadvantages, and challenges of proton therapy and carbon-ion therapy
Radiotherapy, particularly proton therapy and carbon-ion therapy, shows potential advantages in the treatment of head and neck cancer and prostate cancer due to its ability to more precisely concentrate radiation doses on tumor tissue while reducing damage to surrounding healthy tissue. Proton therapy has demonstrated a lower rate of adverse effects in terms of safety, but its efficacy compared to conventional photon therapy remains a matter of debate. Carbon-ion therapy has shown varying results in terms of efficacy and survival rates across studies, indicating the need for further research to confirm its benefits. The widespread application of these two treatment technologies is limited by cost, technical requirements, and availability, but their potential to improve treatment outcomes and reduce side effects continues to drive the medical community to explore and refine these therapeutic methods.
Photothermal and photodynamic therapy
The basic principle of photothermal therapy (PTT) and photodynamic therapy (PDT) involves using photosensitized materials in conjunction with light radiation to target diseases and achieve therapeutic purposes. 374 Upon reaching the tumor lesion and being irradiated, photothermal and photodynamic agents convert light energy into heat or chemical energy to destroy tumor cells. PTT is practical in regulating temperature changes by adjusting the light intensity to control cell death, 375 whereas the key mechanism of PDT is inducing reactive oxygen species (ROS), 376 which is followed by the release of inflammatory mediators and the initiation of an immune response. 377 , 378
Current clinical application
Despite the potential therapeutic value of PTT, clinical studies are still limited. A pilot study explored the safety and efficacy of gold-silica nanoshells (GSNs) in low-intermediate-grade prostate cancer patients. 379 GSNs exhibit maximal absorption in the near-infrared light spectrum and convert light energy into heat. In the 15 enrolled patients, after intravenous administration, GSNs accumulated in the prostate lesions due to the abnormal tumor vasculature, followed by subsequent near-infrared laser illumination. After 12 months of follow-up, prostate biopsies showed that 13 of 15 patients were cancer-free at the ablation zones.
Compared to PTT, PDT has been more extensively studied in clinical trials. A phase I study investigated the safety and efficacy of the photosensitizer Visudyne for treating vertebral metastases, 380 finding significant pain reduction but no improvement in overall survival. Hypericin is a natural compound with phototoxicity to malignant cells. 381 Based on this feature, hypericin is considered as a PDT agent. The FLASH study is a multicenter, placebo-controlled, double-blinded phase III trial that included patients with early-stage mycosis fungoides and cutaneous T-cell lymphoma (MF/CTCL) to examine the therapeutic effect of synthetic hypericin via PDT. 382 The results indicated a higher index lesion response rate (ILRR) in the hypericin group compared to the placebo group, and after three cycles of administration, the ILRR increased to 49%.
The advantages, disadvantages, and challenges of PTT and PDT
PTT and PDT are two optical treatment methods used for diseases such as cancer. PTT converts light energy into heat to destroy cancer cells in a targeted manner, characterized by minimally invasive nature, the capability for repeated applications, and the absence of surgical intervention. However, it requires precise temperature control to prevent thermal damage to surrounding healthy tissues. PDT relies on the selective interaction of photosensitizers with light to eliminate tumor cells, providing high selectivity and the possibility of triggering an immune response. Nevertheless, patients undergoing PDT are susceptible to photosensitivity post-treatment, and the procedure tends to be costly. Both techniques confront restrictions regarding the depth of light penetration, which can impact their efficacy against larger or more deeply located tumors. Despite these hurdles, PTT and PDT continue to hold significant positions in cancer therapy research due to their distinct therapeutic mechanisms and promising clinical utility.
Anti-angiogenesis therapy
Inducing or accessing vasculature through angiogenesis is one of the hallmarks of cancer. 271 Tumor angiogenesis exhibits unique characteristics, including a disordered structure, high permeability, and a lack of pericytes and smooth muscle cells, which differ significantly from angiogenesis under normal physiological conditions, such as embryonic development. 383 The process of tumor angiogenesis is complex, regulated by both malignant and non-malignant cells through various pro-angiogenic and anti-angiogenic molecules and pathways in the tumor microenvironment, 384 thus providing potential therapeutic targets.
Molecular/pathway/cellular network in tumor angiogenesis
The vascular endothelial growth factor (VEGF) family is the group of molecules that has garnered the most research interest in tumor angiogenesis. The VEGF family, encoded by the human genome, consists of five members, 385 among which VEGF-A (previously known as VEGF) is the most crucial mediator for activating endothelial cell reprogramming, proliferation, and migration by binding to VEGF receptor 2 (VEGFR2) during tumor angiogenesis. 386 Upon binding, VEGFR2 becomes dimerized 387 and activates downstream pathways, including RAS, ERK-MAPK, and PI3K-AKT signaling. 388 , 389 , 390 In addition to its interactions with endothelial cells, VEGF-A can also influence immune cells through various mechanisms, including impeding dendritic cell maturation, 391 increasing the infiltration of regulatory T cells, 392 and inducing cytotoxic T cell exhaustion. 393
The epidermal growth factor (EGF) family is another crucial component that contributes to tumor angiogenesis through both direct and indirect effects. For example, EGF promotes endothelial cell proliferation by binding to the EGF receptor (EGFR) and increases the secretion of VEGF by human pulmonary smooth muscle cells. 394 In addition to the EGF family, the family of fibroblast growth factors (FGFs) and their receptors may play a significant role in tumor angiogenesis, as their overexpression has been observed across a wide range of malignancies with prognostic significance 395 ; however, the underlying mechanisms are not yet fully understood.
Translational value in clinical practice and future challenges
Targeting the VEGF-VEGFR signaling pathway is the primary strategy for anti-angiogenic tumor therapy. Bevacizumab was the first FDA-approved monoclonal antibody targeting VEGF-A for colorectal cancer (CRC) therapy and has shown significant improvements in overall and progression-free survival in patients with metastatic CRC. 396 With advancements in basic research and clinical trials, bevacizumab is now used for a broader range of solid malignancies, including ovarian cancer, 397 glioma, 398 hepatocellular carcinoma (HCC), 22 cervical cancer, 11 and renal cell carcinoma. 399
Another monoclonal antibody targeting VEGF-VEGFR, ramucirumab, exerts its effect by blocking VEGFR2. It was initially approved for the treatment of gastric and gastroesophageal junction adenocarcinoma, showing a survival benefit, 400 and subsequently for NSCLC, 401 CRC, 402 and HCC. 403
In addition to monoclonal antibody drugs, tyrosine kinase inhibitors (TKIs) have also attracted research interest due to the extensive involvement of tyrosine kinases in the downstream pathways of VEGFR, such as Sorafenib, 404 , 405 , 406 sunitinib, lenvatinib, and apatinib. 407 , 408 , 409 Also, there are several TKIs directly targeting VEGFR family. Nintedanib is a type of VEGFR-TKI that blocks certain signals inside cells, preventing the growth and spread of fibroblasts. In a phase III trial, it was used with docetaxel to treat advanced NSCLC and significantly improved PFS. 410 Apatinib, another VEGFR-2 inhibitor, has shown a 76.7% disease control rate in treating NSCLC patients who did not respond to initial or follow-up treatments. 411 Fruquintinib, a newer small molecule inhibitor, has also demonstrated promising results, with a 71% increase in progression-free survival compared to a control group in advanced non-squamous NSCLC treatment. 412
The advantages, disadvantages and challenges of anti-angiogenic drugs
Anti-angiogenic drugs effectively inhibit the formation of new blood vessels in tumors, thereby restricting the supply of nutrients and oxygen to cancer cells and slowing down tumor growth. These drugs have a strong targeting ability, with minimal impact on normal cells. When used in combination with other cancer treatments such as chemotherapy and targeted therapies, they can enhance therapeutic effects and delay the development of resistance. However, in some cases, they cause cardiovascular adverse reactions, such as hypertension and heart failure, which presents a limitation in the use of these drugs. Furthermore, although the combination treatment with other drugs is effective, the high cost could be a significant challenge for patients.
Primary and acquired drug resistance poses a significant challenge for anti-angiogenic therapy due to various mechanisms. Liver kinase B1 (LKB1), encoded by STK11, is a key factor in responding to stress conditions like hypoxia. 413 A retrospective study found that the loss or deficiency of LKB1 in NSCLC leads to primary resistance to bevacizumab, possibly due to metabolic reprogramming in tumor cells. 414 Tina et al. used a bevacizumab-resistant mouse xenograft model of human lung cancer and found that upregulated EGFR in stroma led to acquired resistance. 415 To overcome drug resistance, basic studies are needed to explore the unrevealed compensatory adjustments and other changes in the tumor microenvironment after administration of anti-angiogenesis therapy, and to identify targetable molecules for combination therapy.
Nanomedicine
With the approval and market introduction of the first nanomedicine (Doxil) by the FDA in 1995, 416 the application of nanomedicines in cancer treatment began to gain attention. Nanomedicines are an emerging drug delivery system that leverages nanotechnology to enhance the bioavailability, stability, targeting, and therapeutic efficiency of drugs. These medicines typically refer to drug carriers or drug molecules at the nanoscale (1 to 100 nanometers), which can include traditional small-molecule drugs, peptides, proteins, nucleic acids, or other bioactive molecules.
The application of nanomedicines in cancer therapy encompasses a variety of nanocarriers and nanotechnologies. Currently, common nanomedicines used for tumors include lipid-based nanomedicines, inorganic nanomedicines, and polymer-based nanomedicines. Additionally, viral vectors and ADCs (Antibody-Drug Conjugates) can also be considered forms of nanomedicines, which have been discussed earlier.
Lipid Nanomedicines
Liposomes are vesicles formed by the self-assembly of lipids into one or more concentric bilayers (unilamellar or multilamellar) with an enclosed aqueous compartment, ranging in size from 30 nm to the micrometer scale. 417 Leveraging the enhanced permeability and retention (Enhanced Permeability and Retention, EPR) effect, liposome carriers offer the advantages of protecting encapsulated drugs from degradation, extending their half-life, and controlling drug release. By actively or passively delivering drugs to tumor tissues, liposomes contribute to reducing systemic side effects and enhancing cancer treatment. Currently, liposome nanoparticle formulations are among the most widely used nanomedicines. Encapsulating chemotherapy drugs is a common application of liposome nanomedicines, such as Doxorubicin and Paclitaxel. Doxil (liposome-encapsulated Doxorubicin), Myocet (liposome-encapsulated Doxorubicin HCl), and Abraxane (liposome-encapsulated Paclitaxel) have been FDA-approved for treating certain types of cancer, including breast cancer and non-small cell lung cancer. The LNPs mentioned earlier are also a type of lipid nanocarrier.
Solid Lipid Nanoparticles (SLNs) are drug carriers with a solid lipid core and surfactant coating, enhancing stability and drug release control. They can encapsulate both hydrophobic and hydrophilic drugs, offering advantages in cancer treatment. For instance, curcumin-loaded SLNs show potent cytotoxicity against SKBR3 breast cancer cells compared to curcumin alone, 418 while RGD-modified doxorubicin SLNs enhance tumor targeting and therapeutic efficacy. 419
Exosomal nanomedicines, often referred to as natural liposomes, are emerging as a rising star in drug delivery systems. Exosomes are small vesicles released by cells into the extracellular environment, playing a crucial role in cell-to-cell communication and capable of carrying proteins, RNA, DNA, and other biomolecules. Due to their natural characters and biological functions, exosomes have become a promising drug delivery platform. For instance, Rayamajhi et al. revealed that exosomes secreted by macrophages could be used for the delivery of Doxorubicin (a clinical drug) for breast cancer treatment. 420 In the future, liposome nanomedicines are expected to see more clinical translation.
Inorganic nanomedicines
Inorganic nanomedicines, which include metal nanoparticles (such as gold, silver, and iron oxides), quantum dots, carbon-based materials (like carbon nanotubes and graphene), and other particles (mesoporous silica, magnetic nanoparticles, and nanoscale calcium materials), are utilized for cancer diagnosis and treatment. As mentioned earlier, gold nanoparticles serve as a medium for photothermal therapy, killing tumor cells through localized heating. Iron oxide nanoparticles (such as Fe3O4 and γ-Fe2O3) can be used as contrast agents for magnetic resonance imaging (MRI) to enhance tumor visualization. 421 Another kind of nanomedicine uses Titanium dioxide 1 (TiO2) and zinc oxide (ZnO) to generate reactive oxygen species (ROS), 422 which can kill tumor cells under light exposure. Additionally, certain nanomedicines, such as those containing Fe3 + , SiO2, Pt, and Li, can induce various cell death pathways, including ferroptosis, pyroptosis, autophagy, and necroptosis. 423 Certain metal-organic frameworks (MOFs) are capable of encapsulating and releasing therapeutic gases, such as nitric oxide (NO), which has antitumor and anti-angiogenic effects. Silica nanoparticles have been shown to activate the immune system and enhance antitumor immune responses. They can activate immune cells through pattern recognition receptors (such as Toll-like receptors, TLRs), thereby promoting the clearance of tumor cells. Recent reports also suggest that inorganic nanomaterials can induce macrophage polarization. Researchers have developed a hollow mesoporous Prussian blue (HMPB) nanosystem disguised by a macrophage membrane. 424 The released iron ions promoted the differentiation of M1-like tumor-associated macrophages (TAMs) through the interferon regulatory factor 5 (IRF5) pathway, stimulated cytotoxic T cells, and generated an effective antitumor effect. 425 The applications and therapeutic strategies involving inorganic nanomedicines are highly innovative and cover a broad range of approaches.
Polymeric Nanomedicines Polymeric nanoparticles
Polymeric Nanomedicines Polymeric nanoparticles, such as poly(lactic-co-glycolic acid) (PLGA) nanoparticles, are widely used in cancer treatment. These nanoparticles can encapsulate drugs, control their release, and enhance drug targeting. PLGA-packed drugs include both chemotherapy agents and immunosuppressants. Dasharath Chaudhari developed paclitaxel (PTX)-loaded adenosine (ADN)-conjugated PLGA nanoparticles to combat triple-negative breast cancer (TNBC), which were found to be biocompatible and exhibited improved anti-TNBC activity. Another experiment demonstrated that in a subcutaneous model of MC38 colon cancer, 426 PLGA nanoparticle therapy was as effective as treatment with soluble anti-PD-L1 monoclonal antibody (mAb), resulting in significantly reduced tumor growth. 427 In other studies, Chi-Son Chang and colleagues reported that fenbendazole-incorporated PLGA nanoparticles exerted significant anticancer effects in epithelial ovarian cancer cells and xenograft models, including patient-derived xenografts (PDX). 428 PLGA has also been involved in preclinical studies for combination therapy. One study justifies additional preclinical safety trials and the clinical evaluation of 177Lu-PLGA(RGF)-CXCR4L as a potential combined treatment for colorectal cancer. 429 Guen Tae Kim showed that the antitumor effect of the chemotherapy AC regimen (Adriamycin and cyclophosphamide) was increased by cotreatment with 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) in the MDA-MB-231 TNBC xenograft mouse model. 430 Among other polymers, dendrimers, as a special class of polymers, participate in drug transport. G5 PAMAM dendrimers have demonstrated successful folate-receptor-targeted delivery of doxorubicin. 431 Another study reported that dendrimer-functionalized nanoparticles delivering the p53 tumor suppressor gene to the tumor site resulted in an improved antiproliferative effect compared to the free naked gene. 432
Advantages, disadvantages, and challenges
Nanomedicines offer a multitude of benefits over conventional free-form drugs, providing a more effective approach to drug delivery. By enhancing drug solubility, they safeguard medications from early degradation, which in turn boosts stability and bioavailability. They also minimize off-target interactions within the body, extending the drug’s circulation time and improving therapeutic outcomes. Additionally, nanomedicines increase the concentration of drugs at the site of pathology while simultaneously reducing their presence in healthy tissues, thereby mitigating toxic side effects. The controlled release of drugs and their targeted distribution to specific tissues further optimize pharmacokinetic profiles. Moreover, these advanced systems facilitate the penetration of formidable biological barriers, such as the blood-brain barrier, surmounting obstacles that hinder the delivery of therapeutics.
Although inorganic nanomaterials show great potential in various fields, they also have significant drawbacks. Firstly, these materials may have potential biological toxicity. Due to their high surface area, they may interact unpredictably with biomolecules, leading to cell or tissue damage. Secondly, stability issues in biological systems, such as easy aggregation and sedimentation, or coverage by biomolecules, may reduce their therapeutic effectiveness. Additionally, the distribution, metabolism, and clearance mechanisms of these nanomaterials in the body are not fully understood, affecting the assessment of their safety and efficacy. Lastly, the long-term health and environmental risks of inorganic nanomaterials remain unclear, increasing concerns about their widespread application. Therefore, despite the innovative applications that inorganic nanomaterials may bring, their potential risks must be thoroughly researched and strictly managed before they are used clinically and environmentally.
Currently, the translation of nanomedicines has not progressed as rapidly as the numerous positive preclinical results would have suggested. Key issues related to the clinical development of nanoparticle nanomedicines include biological challenges, biocompatibility and safety, large-scale manufacturing, government regulations, intellectual property (IP), and overall cost-effectiveness compared to current therapies. The cost, in particular, has limited the clinical transfer of this approach.
The combination therapy against tumor
Combination therapy in the field of oncology has achieved significant advancements, becoming a key strategy for enhancing treatment efficacy, combating drug resistance, and extending patient survival. Notably, the combination of immune checkpoint inhibitors has demonstrated synergistic effects across various types of cancer, improving therapeutic outcomes. The latest data from the IMbrave150 study show that patients with Hepatocellular Carcinoma (HCC) who received the recommended front-line combination therapy of Atezolizumab and Bevacizumab (T + A) had a mOS of 19.2 months compared to 13.4 months with sorafenib. In the Chinese subgroup of patients, the mOS was extended to two years compared to 11.4 months with sorafenib. Moreover, the ORR for this treatment reached 30%. 400 Additionally, recent findings suggest that “T+A” regimen paired with TACE (Transarterial Chemoembolization) or HAIC (Hepatic Artery Infusion Chemotherapy) may be a significant research direction for large hepatocellular carcinoma (HCC). Hence, the scientifically sound combination of treatments in clinical practice will benefit more cancer patients.
The year 2023 was a fruitful year for research in combination therapies. In March, Novartis announced that the FDA approved the combination therapy of Tafinlar (dabrafenib) and Mekinist (trametinib), making Tafinlar + Mekinist the first approved targeted combination therapy for the treatment of pediatric patients with low-grade gliomas carrying the BRAF V600E mutation (Supplementary Table 3 ). 433 In May, Professor Ruihua Xu’s team’s SPOTLIGHT study on advanced gastric cancer showed that the combination of zolbetuximab and mFOLFOX6 reduced the risk of disease progression or death by 24.9% ( P = 0.0066) compared to the placebo + mFOLFOX6 group, with median progression-free survival (mPFS) of 10.61 months and 8.67 months, respectively. 434 In June of the same year, the clinical trial of talazoparib and enzalutamide for metastatic castration-resistant prostate cancer (mCRPC) in adults was approved for the first time. 435 This combination therapy can reduce the progression and mortality rates by 55% in mCRPC patients with HRR mutations. In November, the JUPITER-02 study, the world’s first clinical study also led by Professor Ruihua Xu’s team comparing first-line immunotherapy combined with chemotherapy to chemotherapy alone in nasopharyngeal carcinoma (NPC), confirmed that the treatment with tislelizumab combined with chemotherapy significantly improved overall survival (OS) for the first-line treatment of recurrent or metastatic NPC, with a 3-year OS rate of 64.5%, and a 37% reduction in the risk of death. 436 This study set a record for OS benefits in patients with advanced NPC. Additionally, a clinical study reported in the New England Journal of Medicine revealed that first-line treatment with osimertinib-chemotherapy led to significantly longer PFS than osimertinib monotherapy among patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC). 437 These studies mark significant advancements in combination therapies for cancer treatment.
Combined therapy with immune checkpoint inhibitors
From 2006 to March 20, 2024, the US Food and Drug Administration has approved 90 combination therapies for cancer (Supplementary Table 3 ). The use of immune checkpoint inhibitors (ICIs) in conjunction with other treatment modalities is noteworthy. The combination of Nivolumab and Ipilimumab has appeared 8 times, covering various cancers including RCC, melanoma, mesothelioma, and NSCLC et al. Combinations with chemotherapy have emerged 16 times, including Pembrolizumab + Chemotherapy, Atezolizumab + Nab-Paclitaxel, Atezolizumab + Carboplatin + Etoposide, and Nivolumab + Chemotherapy. These combination regimens have become primary first-line options for a range of cancers in clinical practice.
In the latest guidelines from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN), the integration of immunotherapy with other treatment approaches has become an essential component of first-line therapy for various cancers. Considering the findings from the 2021 KEYNOTE-826 study, the administration of Pembrolizumab combined with TP (paclitaxel and cisplatin or paclitaxel and carboplatin) along with optional Bevacizumab has markedly enhanced the OS and PFS for patients battling advanced cervical cancer. 438 Following these outcomes, the FDA formally granted approval for this combined treatment approach as the new standard first-line therapy for advanced cervical cancer. The 2023 ASCO meeting presented the 39.1-month follow-up data from the KEYNOTE-826 trial, which indicated that incorporating Pembrolizumab into chemotherapy with or without Bevacizumab significantly lowered the mortality risk by 40% in individuals with a PD-L1 CPS (Combined Positive Score) of 1 or greater, by 37% across the entire studied population, and by 42% in those with a CPS of 10 or higher, while maintaining an acceptable safety profile. For non-small cell lung cancer (NSCLC), Pembrolizumab is recommended for patients with high PD-L1 expression, while for those with low or unknown PD-L1 expression levels, combinations of Nivolumab with Ipilimumab and chemotherapy, or Pemetrexed with platinum-based chemotherapy are recommended. The treatment recommendation for advanced melanoma includes the combination of Nivolumab and Ipilimumab. Based on the clinical trials results from CheckMate 214, 439 KEYNOTE-426, 440 JAVELIN 101, 441 CheckMate 9ER, 442 first-line treatment recommendations for renal cell carcinoma (RCC) include Nivolumab+Ipilimumab, Pembrolizumab+Axitinib, Avelumabas + +Axitinib, well as the combination of Nivolumab+Cabozantinib. based on the results from the KEYNOTE-B61 443 study, the combination of Lenvatinib and Pembrolizumab or Everolimus has shown durable anti-tumor activity in patients with previously untreated non-clear cell RCC (including papillary and chromophobe renal cell carcinoma). The objective response rate (ORR) reached 49%, with 74.6% of patients experiencing responses lasting at least 12 months. The 12-month PFS rate was 63%, and the 12-month overall survival (OS) rate was 82%, indicating promising survival outcomes. These results further support the use of Pembrolizumab and Lenvatinib as a first-line treatment strategy for patients with advanced non-clear cell RCC. For patients with head and neck squamous cell carcinoma (HNSCC), Pembrolizumab alone or in combination with chemotherapy is an option. The first-line treatment recommendation for HCC is the combination of Atezolizumab with Bevacizumab. Pembrolizumab is the first-line treatment choice for colorectal cancer (CRC) with high microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR). For triple-negative breast cancer (TNBC), the first-line treatment recommendation includes the combination of Ipilimumab with chemotherapy.
Combined drug therapy in clinical trials and preclinical basic research for cancer treatment most frequently involves co-administration with immunosuppressants. Inhibitors targeting PD-1 (such as Nivolumab and Pembrolizumab) and inhibitors targeting CTLA-4 (such as Ipilimumab) are star drugs in immunotherapy, and they have been widely studied in the treatment of various solid tumors and hematological cancers. In a phase II/III clinical trial (RELATIVITY-047) examining combination therapy for unresectable or untreated metastatic melanoma, satisfactory improvements are seen with Relatlimab (anti-LAG-3) and Nivolumab (anti-PD-1). With this combination, the 1-year PFS rate increased from 36.0% to 47.7% and the median PFS improved dramatically from 4.6 months to 10.1 months. 444 However, further follow-up is required to assess the ultimate therapeutic efficacy. 444 In preclinical research, combination therapy involving various treatment modalities has been explored. For instance, Guangji Zhang et al. conducted a study combining Nivolumab and GD2 CAR-T treatment in Glioblastoma. This combination exhibited the highest efficacy, leading to an extension of survival in mice with tumor burden for up to 60 days. 445 Another study by Lanqi Gong et al. demonstrated that CD70 blockade, when combined with anti-PD-1 treatment, synergistically reinvigorated T-cell immunity against nasopharyngeal carcinoma. The evaluation of anti-CD70 monotherapy in combination with anti-PD-1 therapy using xenograft-derived organoids and humanized mice showed improved tumor-killing efficacy. 446 Additionally, a recent study reported that PD-1 + IL-2 combination therapy significantly alters the differentiation program of PD-1 + TCF-1+ stem-like CD8 + T cells, which merits consideration as a potential regimen for cancer treatment. 447 Also, clinical trials of combination ICIs have achieved significant results, and more clinical trials are underway. We have listed some representative combination cancer treatment clinical trials in Table 6 . The clinical trials with results showed that patients received more benefits from the combination treatment. Meanwhile, new types of cancer therapy such as oncolytic virus have already started their clinical trials with ICIs.
Combined strategies in small molecular inhibitors
In the FDA-approved combination therapy, the combination of small molecular drugs appeared 22 times, including Neratinib + Capecitabine, Encorafenib + Cetuximab, Tucatinib + Trastuzumab + Capecitabine, Ibrutinib + Rituximab, and Olaparib + Bevacizumab. This indicates that the combined use of small molecule inhibitors is also commonly employed in clinical practice. In the latest clinical treatment guidelines, the combined use of targeted therapy drugs is tailored to specific cancer types and molecular markers, encompassing a variety of different combinations. For instance, in patients with non-small cell lung cancer (NSCLC) harboring EGFR mutations, EGFR inhibitors such as Erlotinib may be combined with anti-angiogenic drugs like Bevacizumab. 448 For NSCLC with ALK rearrangements, ALK inhibitors like Crizotinib may be used in conjunction with other treatment modalities. 449 In the treatment of HER2-positive breast cancer, HER2 inhibitors such as Trastuzumab may be combined with Pertuzumab and chemotherapy. 450 The combination of the BRAF inhibitor Dabrafenib with the MEK inhibitor Trametinib has become the standard treatment for melanoma with BRAF V600E/K mutations. In ovarian and breast cancers with BRCA mutations, PARP inhibitors like Olaparib may be combined with chemotherapy. 451 CDK4/6 inhibitors such as Palbociclib are used in combination with endocrine therapy for hormone receptor-positive breast cancer. 452 Additionally, IDH1/IDH2 inhibitors like Ivosidenib are used in combination with Azacitidine for leukemias and other solid tumors carrying the respective mutations. 453
Due to the wide variety of small molecule inhibitors, its combination with other drugs is very common. While in preclinical research, blocking the oncogene or its signaling pathway by using small molecule inhibitors combined with other treatments is a regular method to test therapeutic efficiency. For example, Liu et al. successfully combined a PI3K inhibitor, the downstream of TROY, with Sorafenib to suppress tumor development in a HCC mouse model. 454 Jiang et al. demonstrated that the combination of ARV-771 and romidepsin keep tumor grew more slowly than HDAC or BET agent alone in ESCC xenograft. 455 The combination of small molecular inhibitor and CRISPR system as a drug screening system to find neoantigen is also another application in basic research. Zheng et al. integrated the screening of a small-molecule inhibitor library and a druggable CRISPR library, revealing that GSK-J4 exhibits synthetic lethality with donafenib in liver cancer. 456
Small molecule inhibitors are frequently incorporated into clinical trials for combination therapies, owing to their lower toxicity profile in humans. One clinical trial showed that the combination of lapatinib plus trastuzumab improved disease-free survival from 1.0 year (lapatinib alone) to 1.9 years, and overall survival from 3.6 to 3.9 years. 457 A clinical trial from the New England Journal of Medicine, showed that in a cohort of 44 patients with rare Colorectal Cancer carrying the Mutated KRAS G12C, the combination therapy of Adagrasib and Cetuximab decreased the percentage of grade 3 or 4 adverse reactions compared to Adagrasib monotherapy and increased the drug response rate from 19% to 46%. 458 These findings indicate the strong efficacy of the combination. 459 These results indicate that the addition of small molecule inhibitors to other treatments can extend patients’ lives.
Combined use of anti-angiogenic drugs
The combination of anti-angiogenic drugs with other treatment regimens is a common clinical practice for medication use. In the FDA approval combination treatment table, the combination of anti-angiogenic therapies is mentioned 14 times, which include: Bevacizumab + Atezolizumab, Ramucirumab + Erlotinib, Axitinib + Pembrolizumab, Lenvatinib + Pembrolizumab, Neratinib + Capecitabine, Tucatinib + Trastuzumab + Capecitabine, and Enfortumab Vedotin-ejfv + Pembrolizumab et al. This suggests the importance of anti-angiogenic drugs as adjuvant therapy for combined anti-tumor therapy.
In the latest clinical guidelines, the combination of anti-angiogenic drugs with other treatment modalities has become part of the first-line treatment regimens for specific cancer patient subgroups. For instance, in non-small cell lung cancer (NSCLC), the combination of Bevacizumab with chemotherapy is recommended for patients without EGFR or ALK gene mutations. 460 In patients with metastatic colorectal cancer (CRC), Bevacizumab is also used in conjunction with chemotherapy, especially for those with tumors of KRAS, NRAS, and BRAF wild-type status. 461 Additionally, for patients with advanced hepatocellular carcinoma (HCC), the combination therapy of Bevacizumab with the immune checkpoint inhibitor Atezolizumab is a first-line option. In the treatment of breast and ovarian cancer, anti-angiogenic drugs may also be combined with chemotherapy or other targeted therapy drugs based on the patient’s hormone receptor status and genetic characteristics. The selection of these combination treatment regimens is based on the analysis of patient biomarkers, genetic testing results, and clinical features to ensure that patients receive the most effective treatment.
The novel mechanism of anti-angiogenesis not only use of anti-VEGF-related products, particularly when combined with other drugs, deserves attention. Kayoko Hosaka et al. discovered how KRAS-mutated epithelial cancers resist anti-angiogenic drugs by using ANG2, overcoming anti-VEGF actions. Combining anti-VEGF and anti-ANG2 treatments showed strong, synergistic effects against these cancers. 462 Another recent study reported that MFGE8 activates the ERK/AKT pathway to promote angiogenesis in esophageal squamous cell carcinoma (ESCC). Blocking the angiogenesis function of MFGE8 with its neutralizing antibody, combined with cisplatin treatment, could minimize the tumor size in ESCC tumor-burden mice [L]. These preclinical studies provide potential treatment ideas for cancer patients.
Bevacizumab, as a hot drug against angiogenesis, has also been involved in various clinical trials. One clinical trials combined Bevacizumab and Erlotinib in phase II advanced ovarian cancer showed the mPFS reached 25.6 months. 463 A recent clinical trial published in the New England Journal of Medicine, based on data from 246 patients with Refractory Metastatic Colorectal Cancer, revealed that the median overall survival in the combination therapy group (Trifluridine-Tipiracil plus Bevacizumab) was 10.8 months, which is greater than the 7.5 months in the Trifluridine-Tipiracil monotherapy group, providing a new direction for the treatment of Refractory Metastatic Colorectal Cancer. 464 Another new drug targeting both VEGFR and EGFR, Rivoceranib, has demonstrated remarkable efficacy in tumor treatment. Clinical trials have indicated that the combination of camrelizumab and rivoceranib significantly improves overall survival and progression-free survival, and provides clinically meaningful benefits for patients with HCC compared to sorafenib alone in a cohort of 543 patients. 414 Given the promising clinical activity observed, further exploration of the combination with anti-angiogenic drugs is warranted as a potential alternative to available treatments.
The advantages, disadvantages, and challenges of combination treatment
Even though combination therapies have received multiple FDA approvals and have advantages in overcoming complex diseases and give patients customizable treatment to against tumor. There are still some shortcomings and challenges. First, cancer patients who meet the criteria for combination therapy may need to be further selected to reap benefits while avoiding potential pitfalls. From the combination therapies approved by the FDA and the guidelines, the entry criteria for combination therapy are relatively strict. For instance, the talazoparib + enzalutamide combination therapy approved in November 2023 is specified for the treatment of patients with HRR gene-mutated metastatic castration-resistant prostate cancer. In the pembrolizumab + chemotherapy combination therapy approved on November 25, 2020; it is explicitly stated that TNBC patients must have PD-L1 expression above a certain threshold (CPS ≥ 10) (Supplementary Table 3 ). Additionally, the combined use of different drugs may not always be compatible, and drug interactions may be affected, with the potential for increased the frequency of adverse events on the patient due to the use of multiple medications. One clinical trial (NCT02660034) reported the occurrence of nausea (63%), fatigue (53%), diarrhea (35%), vomiting (31%), and asymptomatic grade 3–4 hepatic immune-related adverse events (39%) in the cohorts receiving combination treatment with Pamiparib and Tiselizumab. 465 Therefore, the selection of drugs and the determination of drug dosages are crucial. Further animal studies and clinical trials are necessary. Moreover, there is an urgent need for predictive biomarkers to guide the entire treatment process. Since the situation with combination therapies differs from monotherapy, in addition to regular imaging tests, some blood test indicators also need to be clearly redefined to accurately describe the patient’s treatment response. For patients, combination therapies inevitably increase drug costs. In the future, how to reduce the cost of combination therapies will also be an important issue for drug combination strategies.
Conclusions and prospectives
The aim of oncology drug research and development is to provide more effective and targeted treatment methods for suppressing tumor growth and metastasis. In this review, we summarize the progress and application of various oncology drugs, which encompass a range of types and mechanisms. The advantages, disadvantages, challenges, and representative drugs or clinical trials related to cancer treatments discussed herein are compiled in the table below, offering a comprehensive overview and comparison of different strategies (Table 7 ).
Firstly, AI technology will penetrate every aspect of cancer therapy
Current research is primarily focused on integrating AI with healthcare to facilitate automated diagnosis in tissue pathology. In June 2023, a study published in JAMA, assessed the diagnostic performance of GPT-4 with complex medical records. Findings indicated that GPT-4 identified the correct diagnosis as the primary diagnosis in nearly 40% of instances and offered accurate potential diagnoses in two-thirds of the cases. 466 Microsoft’s recently introduced biomedical model, LLaVA-Med, has the ability to deduce patients’ pathological conditions from imaging such as CT and X-ray. In the realm of cervical cancer screening, Huawei, partnering with KingMed Diagnostic, has developed an AI-assisted model that achieves a high specificity rate exceeding 60%, with an interpretation accuracy for negative slides surpassing 99% and a detection rate for positive abnormalities above 99.9%. These advancements mark merely the beginning. The application of AI in the realm of small molecule inhibitors has been discussed, highlighting its role in enhancing drug design, refining manufacturing processes, and forecasting and assessing outcomes. The convergence of oncology big data with deep learning AI offers a distinctive platform, and the field anticipates innovative findings over the coming decade.
The revelations of the biological principles behind cancer heterogeneity based on multi-omics research are guiding new directions in tumor treatment. Spatial omics, TOF mass spectrometry, and CODEX (CO-Detection by IndEXing) are among the omics studies currently underway in preclinical research. These multi-omics technologies combine molecular characterization with spatial resolution, enabling the dissection of tumor molecular structures and the spatial organization of tumor microenvironments. slide-DNA-seq has been applied to tumor samples, allowing for the spatial resolution of genomic data within intact tissue samples. This method has the potential to identify, locate, and characterize individual clones within the three-dimensional tumor structure, thus holding significant promise for elucidating intra-tumor heterogeneity and the evolution of tumor clones during treatment. Spatial proteomics data is also being used to guide clinical trials. Single-cell sequencing, and spatial transcriptomics have been utilized in clinical trials (NCT0604938) to compare the differences in tumor microenvironment components before and after treatment with CD20 CAR-T therapy for B-cell Non-Hodgkin’s Lymphoma. The use of multi-omics data to predict the therapeutic effects of drugs based on tumor molecular data can further drive targeted cancer therapy.
New drugs based on the cancer metabolic adaptation theory
Tumors are not only genetic diseases but also metabolic disorders, as evidenced by the fact that even with ample oxygen supply, tumor cells prefer to undergo glycolysis, promoting lactic acid secretion, known as the “Warburg effect“. 467 In 1948, Sidney Farber described how the anti-folate drug aminopterin inhibits dihydrofolate reductase, thereby blocking the synthesis of nucleic acids and proteins in acute leukemia in children. Aminopterin became the progenitor of a large class of chemotherapeutic drugs. Since that time, research on drugs targeting tumor metabolism has never ceased. Fatty acid metabolism plays a significant role in the growth and metastasis of tumor cells. Fatty acids are involved not only in the structural synthesis and signal transduction of phospholipids on the surface of cancer cell membranes but also maintain energy requirements through β-oxidation and utilize NADPH to maintain redox balance. Lu Zhimin’s team published research indicating that the gluconeogenic enzyme phosphoenolpyruvate carboxykinase 1 (PCK1) has protein kinase activity and phosphorylates protein substrates using GTP as the phosphate donor, continuously stimulating tumor lipid synthesis to promote tumor progression. 468 These data further attracted researchers’ attention to accelerating the development of metabolic-related drugs. However, drugs that block some metabolic pathways can affect the uptake of normal cells, making the development of lipid metabolism drugs exceptionally challenging. For example, clinical development of hexokinase (HK) inhibitors such as 2-deoxyglucose has been halted due to high toxicity or low efficacy. 469 To date, only a limited number of drugs targeting cancer metabolism have been successfully applied clinically (FDA-approved enasidenib and Ivosidenib for acute myeloid leukemia), but this situation may change as our understanding of tumors deepens in the future.
New drugs based on the impact of gut microbiota on tumor progression
The digestive tract is a habitat for bacteria, fungi, and viruses. The occurrence and development of many cancers, as well as the efficacy of their immunotherapy, have been reported to be associated with the gut microbiota. Known intestinal carcinogenic bacteria include Salmonella typhi, which is related to gallbladder cancer, and Helicobacter spp., which is associated with primary liver cancer and gastric cancer. Among them, Helicobacter has been identified as a Group 1 carcinogen by the World Health Organization. In January 2024, Jun Yu’s team reported that, in addition to Helicobacter pylori, a non-H. pylori-driven bacterium, S. anginosus, 470 promotes the occurrence of gastric tumors, which demonstrates the important role of carcinogenic bacteria. Furthermore, in the research on gut microbiota and immunotherapy, two pioneering studies by the teams of Laurence Zitvogel and Giorgio Trinchieri demonstrated that complete gut microbiota is crucial for activating the innate and adaptive immune systems’ effectiveness against three types of cancer therapies. Zitvogel, Gajewski, and Jennifer Wargo published three parallel studies showing that gut commensal bacteria determine the efficacy of anti-PD-1 ICIs in patients with melanoma and epithelial tumors. These results further revealed the influence of carcinogenic bacteria on the composition of primary resistance to immune therapy. In summary, recent research has shown that gut microbiota is closely related to the occurrence and progression of tumors, and studies on gut microbiota are essential for the prevention and treatment of cancer.
Overall, the future of cancer treatment will be diversified and integrated, combining traditional therapeutic methods with emerging technologies to offer patients a wider range of treatment choices. As our understanding of the complexity of cancer increases, we will be better equipped to tackle this challenge and ultimately achieve the control and cure of cancer.
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca. Cancer J. Clin. 71 , 209–249 (2021).
Article PubMed Google Scholar
Worldwide cancer statistics. Cancer Research UK https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer (2015).
Frankel, R. I. Centennial of Röntgen’s discovery of x-rays. West. J. Med. 164 , 497–501 (1996).
CAS PubMed PubMed Central Google Scholar
Steinberg, F. M. & Raso, J. Biotech pharmaceuticals and biotherapy: an overview. J. Pharm. Pharm. Sci. 1 , 48–59 (1998).
CAS PubMed Google Scholar
Commissioner, O. of the. Drug Therapeutics & Regulation in the U.S. FDA https://www.fda.gov/about-fda/fda-history-exhibits/drug-therapeutics-regulation-us (2023).
Grillo-López, A. J. et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr. Pharm. Biotechnol. 1 , 1–9 (2000).
Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 22 , 101–126 (2023).
Article CAS PubMed Google Scholar
Sun, X. et al. Imatinib induces ferroptosis in gastrointestinal stromal tumors by promoting STUB1-mediated GPX4 ubiquitination. Cell Death Dis. 14 , 839 (2023).
Article CAS PubMed PubMed Central Google Scholar
Sun, C., Gao, W., Liu, J., Cheng, H. & Hao, J. FGL1 regulates acquired resistance to Gefitinib by inhibiting apoptosis in non-small cell lung cancer. Respir. Res. 21 , 210 (2020).
Carter, J. & Tadi, P. Erlotinib. in StatPearls (StatPearls Publishing, Treasure Island (FL), 2024).
Tewari, K. S. et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390 , 1654–1663 (2017).
Hietanen, E., Koivu, M. K. A. & Susi, P. Cytolytic properties and genome analysis of Rigvir® Oncolytic Virotherapy virus and other Echovirus 7 Isolates. Viruses 14 , 525 (2022).
Sun, H. et al. TOPK/PBK is phosphorylated by ERK2 at serine 32, promotes tumorigenesis and is involved in sorafenib resistance in RCC. Cell Death Dis. 13 , 450 (2022).
Myers, R. M. et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T cells in CAR-Naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 39 , 3044–3055 (2021).
Article CAS Google Scholar
Bouffet, Eric et al. Dabrafenib plus Trametinib in Pediatric Glioma with BRAF V600 mutations. N. Engl. J. Med. 389 , 1108–1120 (2023).
Carbone David, P. et al. First-line Nivolumab in Stage IV or recurrent non–small-cell lung cancer. N. Engl. J. Med. 376 , 2415–2426 (2017).
Harrington, K. J. et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the Phase III KEYNOTE-048 Study. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 41 , 790–802 (2023).
Joura Elmar, A. et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N. Engl. J. Med. 372 , 711–723 (2015).
Zhang, T. et al. Talimogene Laherparepvec (T-VEC): A review of the recent advances in cancer therapy. J. Clin. Med. 12 , 1098 (2023).
Todo, T. et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat. Med. 28 , 1630–1639 (2022).
Skoulidis, F. et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384 , 2371–2381 (2021).
Finn, R. S. et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382 , 1894–1905 (2020).
Durante, M., Debus, J. & Loeffler, J. S. Physics and biomedical challenges of cancer therapy with accelerated heavy ions. Nat. Rev. Phys. 3 , 777–790 (2021).
Global Anti Tumor Drugs Market Research Report 2023 . https://www.marketresearch.com/Bosson-Research-v4252/Global-Anti-Tumor-Drugs-Research-34934738/ (2023).
Falzone, L., Salomone, S. & Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 9 , 1300 (2018).
Lemberg, K. M., Gori, S. S., Tsukamoto, T., Rais, R. & Slusher, B. S. Clinical development of metabolic inhibitors for oncology. J. Clin. Invest. 132 , e148550 (2022).
Article PubMed PubMed Central Google Scholar
Moreno, C., Muñoz, C., Terol, M. J., Hernández-Rivas, J.-Á. & Villanueva, M. Restoration of the immune function as a complementary strategy to treat Chronic Lymphocytic Leukemia effectively. J. Exp. Clin. Cancer Res. 40 , 321 (2021).
Sliwoski, G., Kothiwale, S., Meiler, J. & Lowe, E. W. Computational methods in drug discovery. Pharmacol. Rev. 66 , 334–395 (2014).
Benjin, X. & Ling, L. Developments, applications, and prospects of cryo‐electron microscopy. Protein Sci. Publ. Protein Soc. 29 , 872–882 (2020).
Article Google Scholar
Tsukamoto, Y. et al. 150-kD oxygen-regulated protein is expressed in human atherosclerotic plaques and allows mononuclear phagocytes to withstand cellular stress on exposure to hypoxia and modified low density lipoprotein. J. Clin. Invest. 98 , 1930–1941 (1996).
Hu, Y. et al. NMR-based methods for protein analysis. Anal. Chem. 93 , 1866–1879 (2021).
Banerjee, A., Bhakta, S. & Sengupta, J. Integrative approaches in cryogenic electron microscopy: Recent advances in structural biology and future perspectives. iScience 24 , 102044 (2021).
Dhakal, A., Gyawali, R., Wang, L. & Cheng, J. A large expert-curated cryo-EM image dataset for machine learning protein particle picking. Sci. Data 10 , 392 (2023).
Skalidis, I. et al. Cryo-EM and artificial intelligence visualize endogenous protein community members. Struct. Lond. Engl. 30 , 575–589.e6 (2022).
CAS Google Scholar
Al-Azzawi, A., Ouadou, A., Tanner, J. J. & Cheng, J. AutoCryoPicker: An unsupervised learning approach for fully automated single particle picking in Cryo-EM images. BMC Bioinforma. 20 , 326 (2019).
Yu, H., Ma, H., Yang, K., Zhao, Y. & Jin, Y. DeepEM: Deep Neural Networks Model Recovery through EM Side-Channel Information Leakage. in 2020 IEEE International Symposium on Hardware Oriented Security and Trust (HOST) 209–218 (2020). https://doi.org/10.1109/HOST45689.2020.9300274 .
Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2023 update. Pharmacol. Res. 187 , 106552 (2023).
Iqbal, N. & Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014 , 1–9 (2014).
Karnoub, A. E. & Weinberg, R. A. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 9 , 517–531 (2008).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503 , 548–551 (2013).
Jänne, P. A. et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 387 , 120–131 (2022).
Ferdinandos, S. et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med . 384 , 2371–2381 (2021).
Araujo, L. H. et al. Molecular profile of KRAS G12C-mutant colorectal and non-small-cell lung cancer. BMC Cancer 21 , 193 (2021).
Qiu, J., Chen, K., Zhong, C., Zhu, S. & Ma, X. Network-based protein-protein interaction prediction method maps perturbations of cancer interactome. PLOS Genet. 17 , e1009869 (2021).
Dang, C. V., Reddy, E. P., Shokat, K. M. & Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 17 , 502–508 (2017).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384 , 2382–2393 (2021).
Hansen, A. L., Xiang, X., Yuan, C., Bruschweiler-Li, L. & Brüschweiler, R. Excited-state observation of active K-Ras reveals differential structural dynamics of wild-type versus oncogenic G12D and G12C mutants. Nat. Struct. Mol. Biol. 30 , 1446–1455 (2023).
Cook, J. H., Melloni, G. E. M., Gulhan, D. C., Park, P. J. & Haigis, K. M. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat. Commun. 12 , 1808 (2021).
Lavoie, H., Gagnon, J. & Therrien, M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 21 , 607–632 (2020).
Research, C. for D. E. and. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc (2021).
Kim, D. et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature 619 , 160–166 (2023).
Changeux, J.-P. & Christopoulos, A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell 166 , 1084–1102 (2016).
Hughes Timothy, P. et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase inhibitor failure. N. Engl. J. Med. 381 , 2315–2326 (2019).
Larkin, J. et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 371 , 1867–1876 (2014).
Chen, Y.-N. P. et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 535 , 148–152 (2016).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11 , 611–617 (2015).
Smith, A. R., Pucheault, M., Tae, H. S. & Crews, C. M. Targeted intracellular protein degradation induced by a small molecule: en route to chemical proteomics. Bioorg. Med. Chem. Lett. 18 , 5904–5908 (2008).
Article PubMed Central Google Scholar
Zhang, X., Crowley, V. M., Wucherpfennig, T. G., Dix, M. M. & Cravatt, B. F. Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. Nat. Chem. Biol. 15 , 737–746 (2019).
Winter, G. E. et al. Selective target protein degradation via Phthalimide conjugation. Science 348 , 1376–1381 (2015).
Han, X. et al. Discovery of highly potent and efficient PROTAC degraders of Androgen Receptor (AR) by employing weak binding affinity VHL E3 Ligase ligands. J. Med. Chem. 62 , 11218–11231 (2019).
Zhao, Q., Lan, T., Su, S. & Rao, Y. Induction of apoptosis in MDA-MB-231 breast cancer cells by a PARP1-targeting PROTAC small molecule. Chem. Commun. Camb. Engl. 55 , 369–372 (2019).
Hines, J., Lartigue, S., Dong, H., Qian, Y. & Crews, C. M. MDM2-recruiting PROTAC offers superior, synergistic anti-proliferative activity via simultaneous degradation of BRD4 and stabilization of p53. Cancer Res 79 , 251–262 (2019).
Arvinas PROTAC® Protein Degrader Bavdegalutamide (ARV-110) Continues to Demonstrate Clinical Benefit in Men with Metastatic Castration-Resistant Prostate Cancer | Arvinas. https://ir.arvinas.com/news-releases/news-release-details/arvinas-protacr-protein-degrader-bavdegalutamide-arv-110 .
Rathkopf, D. E. et al. First-in-human phase 1 study of CC-94676, a first-in-class androgen receptor (AR) ligand-directed degrader (LDD), in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 42 , 134 (2024).
A Study of AC176 for the Treatment of Metastatic Castration Resistant Prostate Cancer - Full Text View - ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05241613 .
A Study to Assess the Safety, Pharmacokinetics, and Anti-Tumor Activity of Oral HP518 in Patients With Metastatic Castration-Resistant Prostate Cancer - Full Text View - ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05252364 .
Petrylak, D. P. et al. A phase 2 expansion study of ARV-766, a PROTAC androgen receptor (AR) degrader, in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 41 , TPS290 (2023).
Arvinas and Pfizer Announce PROTAC® Protein Degrader ARV-471 Continues to Demonstrate Encouraging Clinical Benefit Rate in Patients with Locally Advanced or Metastatic ER+/HER2- Breast Cancer | Pfizer. https://www.pfizer.com/news/press-release/press-release-detail/arvinas-and-pfizer-announce-protacr-protein-degrader-arv .
Lloyd, M. R., Wander, S. A., Hamilton, E., Razavi, P. & Bardia, A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther. Adv. Med. Oncol. 14 , 17588359221113694 (2022).
Mato, A. R. et al. NX-2127-001, a First-in-Human Trial of NX-2127, a Bruton’s Tyrosine Kinase-targeted protein degrader, in patients with relapsed or refractory chronic Lymphocytic Leukemia and B-cell malignancies. Blood 140 , 2329–2332 (2022).
Robbins, D. W. et al. Nx-5948, a selective degrader of BTK with activity in preclinical models of hematologic and brain malignancies. Blood 138 , 2251 (2021).
Wang, H. et al. P1219: BGB-16673, A BTK degrader, overcomes on-target resistance from btk inhibitors and presents sustainable long-term tumor regression in Lymphoma Xenograft Models. HemaSphere 7 , e24358c2 (2023).
Li, J. et al. Abstract CT128: Phase 1 study of HSK29116, a Bruton tyrosine kinase (BTK) proteolysis-targeting chimera (PROTAC) agent, in patients with relapsed or refractory B-cell malignancies. Cancer Res 83 , CT128 (2023).
Khan, S. et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 25 , 1938–1947 (2019).
Study of Oral MRT-2359 in Selected Cancer Patients - Full Text View - ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05546268 .
Salarius Pharmaceuticals, LLC. A Phase 1, Open-Label, Multicenter, First-in-Human Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of SP-3164 in Patients With Relapsed/Refractory Non-Hodgkin’s Lymphoma . https://clinicaltrials.gov/study/NCT05979857 (2023).
Ikena Oncology. A First-in-Human (FiH) Study of IK-595, an Oral Dual MEK/RAF Inhibitor, in Patients With RAS-or RAF-Altered Advanced Solid Tumors . https://clinicaltrials.gov/study/NCT06270082 (2024).
Gao, J., Li, H.-R., Jin, C., Jiang, J.-H. & Ding, J.-Y. Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer. Clin. Transl. Oncol. Publ. Fed. Span. Oncol. Soc. Natl Cancer Inst. Mex. 21 , 1287–1301 (2019).
Vecchio, I., Tornali, C., Bragazzi, N. L. & Martini, M. The Discovery of Insulin: An important milestone in the history of medicine. Front. Endocrinol. 9 , 613 (2018).
Bruce Merrifield. Solid Phase Synthesis, Nobel Lecture. (1984).
Li, J. & Zhu, Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol. Sin. 31 , 1198–1207 (2010).
Akbarian, M., Khani, A., Eghbalpour, S. & Uversky, V. N. Bioactive peptides: synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 23 , 1445 (2022).
Jiang, L. et al. 111In-labeled cystine-knot peptides based on the Agouti-related protein for targeting tumor angiogenesis. J. Biomed. Biotechnol. 2012 , 368075 (2012).
Breeman, W. A. P. et al. Radiolabelling DOTA-peptides with 68Ga. Eur. J. Nucl. Med. Mol. Imaging 32 , 478–485 (2005).
Liu, Z. et al. Two 90 Y-labeled multimeric RGD peptides RGD4 and 3PRGD2 for integrin targeted radionuclide therapy. Mol. Pharm. 8 , 591–599 (2011).
Hofman, M. S. et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet Lond. Engl. 397 , 797–804 (2021).
Lei, Z. et al. Novel peptide GX1 inhibits angiogenesis by specifically binding to transglutaminase-2 in the tumorous endothelial cells of gastric cancer. Cell Death Dis . 9 , 579 (2018).
Chen, K. et al. Evaluation of 64Cu labeled GX1: a phage display peptide probe for PET imaging of tumor vasculature. Mol. Imaging Biol. 14 , 96–105 (2012).
Jiang, J. et al. Noninvasive evaluation of PD-L1 expression using Copper 64 labeled peptide WL12 by micro-PET imaging in Chinese hamster ovary cell tumor model. Bioorg. Med. Chem. Lett. 40 , 127901 (2021).
Dolgin, E. Radioactive drugs emerge from the shadows to storm the market. Nat. Biotechnol. 36 , 1125–1127 (2018).
Guha, M. Amgen swallows Onyx whole. Nat. Biotechnol. 31 , 859–860 (2013).
Kaisary, A. V., Tyrrell, C. J., Peeling, W. B. & Griffiths, K. Comparison of LHRH Analogue (Zoladex) with Orchiectomy in patients with metastatic prostatic carcinoma. Br. J. Urol. 67 , 502–508 (1991).
Lamers, C. Overcoming the shortcomings of peptide-based therapeutics. Future Drug Discov. 4 , FDD75 (2022).
Keating, G. M. Rituximab: A review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-Cell lymphoma. Drugs 70 , 1445–1476 (2010).
Lu, R.-M. et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27 , 1 (2020).
Swain, S. M. et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372 , 724–734 (2015).
Van Cutsem, E. et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 29 , 2011–2019 (2011).
Rugo, H. S. et al. Margetuximab Versus Trastuzumab in patients with previously treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final overall survival results from a randomized Phase 3 trial. J. Clin. Oncol. 41 , 198–205 (2023).
Cortés, J. et al. Pertuzumab Monotherapy after Trastuzumab-based treatment and subsequent reintroduction of Trastuzumab: Activity and tolerability in patients with advanced human epidermal growth factor Receptor 2–positive breast cancer. J. Clin. Oncol. 30 , 1594–1600 (2012).
Goldstein, D. A. et al. First- and second-line Bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a united states–based cost-effectiveness analysis. J. Clin. Oncol. 33 , 1112–1118 (2015).
Townsend, W. et al. Obinutuzumab Versus Rituximab Immunochemotherapy in previously untreated iNHL: Final results from the GALLIUM Study. HemaSphere 7 , e919 (2023).
Warner, J. L. & Arnason, J. E. Alemtuzumab use in relapsed and refractory chronic lymphocytic leukemia: a history and discussion of future rational use. Ther. Adv. Hematol. 3 , 375–389 (2012).
Fisher, J. G. et al. XPO1 inhibition sensitises CLL cells to NK cell mediated cytotoxicity and overcomes HLA-E expression. Leukemia 37 , 2036–2049 (2023).
Tawbi Hussein, A. et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med . 386 , 24–34 (2022).
Mathieu, L. et al. FDA Approval Summary: Atezolizumab and Durvalumab in Combination with Platinum‐Based Chemotherapy in Extensive Stage Small Cell Lung Cancer. Oncologist 26 , 433–438 (2021).
McDermott, D., Haanen, J., Chen, T.-T., Lorigan, P. & O’Day, S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann. Oncol. 24 , 2694–2698 (2013).
Sangro, B. et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol . 35 , 448–457 (2024).
Moroney, J. et al. 158P Triple blockade of the DNAM-axis with COM701 + BMS-986207 + nivolumab demonstrates preliminary antitumor activity in patients with platinum-resistant OVCA. Immuno-Oncol. Technol . 16 , (2022).
An Investigational Immunotherapy Study of BMS-986258 Alone and in Combination With Nivolumab in Participants With Solid Cancers That Are Advanced or Have Spread - Full Text View - ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT03446040 .
Incyte Corporation. An Umbrella Study of INCMGA00012 Alone and in Combination With Other Therapies in Participants With Advanced or Metastatic Endometrial Cancer Who Have Progressed on or After Platinum-Based Chemotherapy (POD1UM-204) . https://clinicaltrials.gov/study/NCT04463771 (2024).
Jiangsu HengRui Medicine Co., Ltd. A Phase I, Multicenter, Open-Label Study of SHR-1702 in Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome . https://clinicaltrials.gov/study/NCT04443751 (2023).
Desai, J. et al. Bgb-A425, an investigational anti-TIM-3 monoclonal antibody, in combination with tislelizumab, an anti-PD-1 monoclonal antibody, in patients with advanced solid tumors: A phase I/II trial in progress. J. Clin. Oncol. 38 , TPS3146 (2020).
Ascierto, P. A. et al. Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann. Oncol. 28 , v611–v612 (2017).
Dumbrava, E. et al. 478 COM701 in combination with BMS-986207 (anti-TIGIT antibody) and nivolumab – preliminary results of safety, tolerability and pharmacokinetics in patients with advanced solid tumors (NCT04570839). J. Immunother. Cancer 9 , A508–A508 (2021).
Liu, Y. et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct. Target. Ther. 8 , 1–42 (2023).
PubMed PubMed Central Google Scholar
Li, S. et al. IMM47, a humanized monoclonal antibody that targets CD24, exhibits exceptional anti-tumor efficacy by blocking the CD24/Siglec-10 interaction and can be used as monotherapy or in combination with anti-PD1 antibodies for cancer immunotherapy. Antib. Ther . 6 , 240–252 (2023).
DeFrancesco, L. Pheast Therapeutics: Another bite at the apple. Nat. Biotechnol . https://doi.org/10.1038/d41587-023-00015-7 . (2023)
Inc, O. OncoC4 to Present Positive Data from Ongoing Phase 1/2 PRESERVE-001 Trial of ONC-392 in Combination with pembrolizumab at SITC 2022. GlobeNewswire News Room https://www.globenewswire.com/news-release/2022/11/07/2549654/0/en/OncoC4-to-Present-Positive-Data-from-Ongoing-Phase-1-2-PRESERVE-001-Trial-of-ONC-392-in-Combination-with-pembrolizumab-at-SITC-2022.html (2022).
Chames, P., Van Regenmortel, M., Weiss, E. & Baty, D. Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 157 , 220–233 (2009).
Rodríguez-Nava, C. et al. Mechanisms of action and limitations of monoclonal antibodies and Single Chain Fragment Variable (scFv) in the treatment of cancer. Biomedicines 11 , 1610 (2023).
Price, T. J. et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 15 , 569–579 (2014).
Lou, H. & Cao, X. Antibody variable region engineering for improving cancer immunotherapy. Cancer Commun. Lond. Engl. 42 , 804–827 (2022).
Shimazaki, K. et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 14 , 7367–7377 (2008).
Holliger, P. & Hudson, P. J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 23 , 1126–1136 (2005).
Alderson, R. F. et al. Characterization of a CC49-based single-chain fragment-beta-lactamase fusion protein for antibody-directed enzyme prodrug therapy (ADEPT). Bioconjug. Chem. 17 , 410–418 (2006).
Peters, C. & Brown, S. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 35 , e00225 (2015).
De Taeye, S. W. et al. FcγR binding and ADCC Activity of human IgG Allotypes. Front. Immunol. 11 , 740 (2020).
Wang, Z., Li, H., Gou, L., Li, W. & Wang, Y. Antibody–drug conjugates: Recent advances in payloads. Acta Pharm. Sin. B 13 , 4025–4059 (2023).
Takegawa, N. et al. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 141 , 1682–1689 (2017).
axispharm. Antibody-drug conjugates(ADCs) list Approved by FDA(2000-2023). AxisPharm https://axispharm.com/antibody-drug-conjugatesadcs-list-approved-by-fda2000-2022/ (2022).
Jin, Y., Schladetsch, M. A., Huang, X., Balunas, M. J. & Wiemer, A. J. Stepping forward in antibody-drug conjugate development. Pharmacol. Ther. 229 , 107917 (2022).
Baah, S., Laws, M. & Rahman, K. M. Antibody–drug conjugates—a tutorial review. Molecules 26 , 2943 (2021).
Tong, J. T. W., Harris, P. W. R., Brimble, M. A. & Kavianinia, I. An Insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules 26 , 5847 (2021).
Ng, G., Spreter, T., Davies, R. & Wickman, G. ZW38, a Novel Azymetric bispecific CD19-directed CD3 T cell engager antibody drug conjugate with controlled T cell activation and improved B cell cytotoxicity. Blood 128 , 1841 (2016).
Andreev, J. et al. Bispecific antibodies and Antibody–Drug Conjugates (ADCs) bridging HER2 and Prolactin Receptor improve efficacy of HER2 ADCs. Mol. Cancer Ther. 16 , 681–693 (2017).
Sellmann, C. et al. Balancing selectivity and efficacy of bispecific Epidermal Growth Factor Receptor (EGFR) × c-MET antibodies and antibody-drug conjugates. J. Biol. Chem. 291 , 25106–25119 (2016).
Sadiki, A. et al. Site-specific conjugation of native antibody. Antib. Ther. 3 , 271–284 (2020).
Samantasinghar, A. et al. A comprehensive review of key factors affecting the efficacy of antibody drug conjugate. Biomed. Pharmacother. 161 , 114408 (2023).
Amani, N., Dorkoosh, F. A. & Mobedi, H. ADCs, as novel revolutionary weapons for providing a step forward in targeted therapy of malignancies. Curr. Drug Deliv. 17 , 23–51 (2020).
Tarantino, P., Ricciuti, B., Pradhan, S. M. & Tolaney, S. M. Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nat. Rev. Clin. Oncol. 20 , 558–576 (2023).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11 , 69 (2021).
Tokarew, N., Ogonek, J., Endres, S., Von Bergwelt-Baildon, M. & Kobold, S. Teaching an old dog new tricks: next-generation CAR T cells. Br. J. Cancer 120 , 26–37 (2019).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21 , 145–161 (2021).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13 , 227–242 (2013).
Hirabayashi, K. et al. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2 , 904–918 (2021).
Munshi, N. C. et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 384 , 705–716 (2021).
Cronk, R. J., Zurko, J. & Shah, N. N. Bispecific chimeric antigen receptor T cell therapy for B cell malignancies and multiple myeloma. Cancers 12 , 2523 (2020).
Spiegel, J. Y. et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 27 , 1419–1431 (2021).
Gardner, R. A. et al. Efficacy of SCRI-CAR19x22 T cell product in B-ALL and persistence of anti-CD22 activity. J. Clin. Oncol. 38 , 3035 (2020).
Chen, X. et al. CD19/BCMA dual-targeting Fastcar-T GC012F for patients with relapsed/refractory B-cell non-Hodgkin’s Lymphoma: An update. Blood 142 , 6847 (2023).
Shah, N. N. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med. 26 , 1569–1575 (2020).
Dai, H. et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J. Hematol. Oncol. J. Hematol. Oncol. 13 , 30 (2020).
Tong, C. et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood 136 , 1632–1644 (2020).
Kotch, C., Barrett, D. & Teachey, D. T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev. Clin. Immunol. 15 , 813–822 (2019).
Bajwa, A. K. et al. Efficacy of Siltuximab for chimeric antigen receptor T-cell therapy toxicities - a multicenter retrospective analysis. Blood 142 , 4502 (2023).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378 , 439–448 (2018).
Fitzgerald, J. C. et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 45 , e124–e131 (2017).
Jaeger, U. et al. Portia: A Phase 1b study evaluating safety and efficacy of Tisagenlecleucel and Pembrolizumab in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood 134 , 5325 (2019).
Choy, E. H. et al. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 16 , 335–345 (2020).
Hamilton, J. A. GM-CSF in inflammation. J. Exp. Med. 217 , e20190945 (2020).
Lee, K. M., Achuthan, A. A. & Hamilton, J. A. GM-CSF: A promising target in inflammation and autoimmunity. ImmunoTargets Ther. 9 , 225–240 (2020).
Martinez, M. & Moon, E. K. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 10 , 128 (2019).
Xie, C. et al. A phase I study of GPC3 targeted CAR-T cell therapy in advanced GPC3-expressing hepatocellular carcinoma (HCC). J. Clin. Oncol 41 , TPS624 (2023).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28 , 1189–1198 (2022).
Kankeu Fonkoua, L. A., Sirpilla, O., Sakemura, R., Siegler, E. L. & Kenderian, S. S. CAR T cell therapy and the tumor microenvironment: Current challenges and opportunities. Mol. Ther. - Oncolytics 25 , 69–77 (2022).
Grosser, R., Cherkassky, L., Chintala, N. & Adusumilli, P. S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36 , 471–482 (2019).
Kuryk, L. et al. Novel insights into mesothelioma therapy: emerging avenues and future prospects. Front. Oncol. 12 , 916839 (2022).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21 , 524–529 (2015).
Atsavapranee, E. S., Billingsley, M. M. & Mitchell, M. J. Delivery technologies for T cell gene editing: Applications in cancer immunotherapy. EBioMedicine 67 , 103354 (2021).
Kamali, E., Rahbarizadeh, F., Hojati, Z. & Frödin, M. CRISPR/Cas9-mediated knockout of clinically relevant alloantigenes in human primary T cells. BMC Biotechnol. 21 , 9 (2021).
Sackett, S. D. et al. Genetic engineering of immune evasive stem cell-derived islets. Transpl. Int. 35 , 10817 (2022).
Rosenberg, S. A. et al. Use of tumor-infiltrating Lymphocytes and Interleukin-2 in the immunotherapy of patients with metastatic melanoma. N. Engl. J. Med. 319 , 1676–1680 (1988).
Khawar, M. B. & Sun, H. CAR-NK cells: From natural basis to design for kill. Front. Immunol. 12 , 707542 (2021).
Du, N., Guo, F., Wang, Y. & Cui, J. NK cell therapy: a rising star in cancer treatment. Cancers 13 , 4129 (2021).
Xie, G. et al. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 59 , 102975 (2020).
Chen, X., Jiang, L. & Liu, X. Natural killer cells: the next wave in cancer immunotherapy. Front. Immunol. 13 , 954804 (2022).
Pang, Z., Wang, Z., Li, F., Feng, C. & Mu, X. Current progress of CAR-NK therapy in cancer treatment. Cancers 14 , 4318 (2022).
Marin, D. et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 30 , 772–784 (2024).
Heipertz, E. L. et al. Current perspectives on “Off-The-Shelf” allogeneic NK and CAR-NK cell therapies. Front. Immunol. 12 , 732135 (2021).
Tang, Y., Zhang, A. X. J., Chen, G., Wu, Y. & Gu, W. Prognostic and therapeutic TILs of cervical cancer—Current advances and future perspectives. Mol. Ther. - Oncolytics 22 , 410–430 (2021).
Dafni, U. et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann. Oncol. 30 , 1902–1913 (2019).
Chandran, S. S. et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 18 , 792–802 (2017).
Sarnaik, A. A. et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 39 , 2656–2666 (2021).
Rohaan, M. W. et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 387 , 2113–2125 (2022).
Alberts, B. Molecular Biology of the Cell . (Garland science, 2017).
Shah, K., Al-Haidari, A., Sun, J. & Kazi, J. U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target. Ther. 6 , 412 (2021).
Baulu, E., Gardet, C., Chuvin, N. & Depil, S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci. Adv. 9 , eadf3700 (2023).
D’Angelo, S. P. et al. SPEARHEAD-1: A phase 2 trial of afamitresgene autoleucel (Formerly ADP-A2M4) in patients with advanced synovial sarcoma or myxoid/round cell liposarcoma. J. Clin. Oncol. 39 , 11504 (2021).
Nagarsheth, N. B. et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 27 , 419–425 (2021).
Sloas, C., Gill, S. & Klichinsky, M. Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors. Front. Immunol. 12 , 783305 (2021).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38 , 947–953 (2020).
Cheng, K. et al. Tumor‐associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun. 42 , 1112–1140 (2022).
Lei, A. et al. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat. Immunol. 25 , 102–116 (2024).
Zhang, L. et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. J. Hematol. Oncol. 13 , 153 (2020).
Wang, X. et al. Metabolic Reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors. Nat. Commun. 14 , 5778 (2023).
Xue, D. et al. Induced pluripotent stem cell-derived engineered T cells, natural killer cells, macrophages, and dendritic cells in immunotherapy. Trends Biotechnol. 41 , 907–922 (2023).
Jirikowski, G. F., Sanna, P. P., Maciejewski-Lenoir, D. & Bloom, F. E. Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA. Science 255 , 996–998 (1992).
Annunziata, C. M. et al. Feasibility and preliminary safety and efficacy of first-in-human intraperitoneal delivery of MCY-M11, anti-human-mesothelin CAR mRNA transfected into peripheral blood mononuclear cells, for ovarian cancer and malignant peritoneal mesothelioma. J. Clin. Oncol. 38 , 3014 (2020).
Reiss, K. A. et al. A phase 1, first-in-human (FIH) study of the anti-HER2 CAR macrophage CT-0508 in subjects with HER2 overexpressing solid tumors. J. Clin. Oncol. 40 , 2533 (2022).
Kang, D. D., Li, H. & Dong, Y. Advancements of in vitro transcribed mRNA (IVT mRNA) to enable translation into the clinics. Adv. Drug Deliv. Rev. 199 , 114961 (2023).
Miller, J. C. et al. Enhancing gene editing specificity by attenuating DNA cleavage kinetics. Nat. Biotechnol. 37 , 945–952 (2019).
Mangeot, P. E. et al. Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nat. Commun. 10 , 45 (2019).
Chen, W. C. W. et al. A synthetic transcription platform for programmable gene expression in mammalian cells. Nat. Commun . 13 , 6167 (2022).
Lundin, J. I. & Checkoway, H. Endotoxin and cancer. Environ. Health Perspect. 117 , 1344–1350 (2009).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17 , 147–167 (2020).
Morgan, M. A., Lange, L. & Schambach, A. Prime time for base editing in the mitochondria. Signal Transduct. Target. Ther . 7 , 213 (2022).
Kim, Y. G., Cha, J. & Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc. Natl Acad. Sci. USA 93 , 1156–1160 (1996).
Ichikawa, D. M. et al. A universal deep-learning model for zinc finger design enables transcription factor reprogramming. Nat. Biotechnol. 41 , 1117–1129 (2023).
Paschon, D. E. et al. Diversifying the structure of zinc finger nucleases for high-precision genome editing. Nat. Commun. 10 , 1133 (2019).
Kaina, B. A genome-wide screening for DNA repair genes: much more players than hitherto known. Signal Transduct. Target. Ther. 5 , 204 (2020).
Ding, W. et al. Zinc finger nucleases targeting the human papillomavirus E7 oncogene induce E7 disruption and a transformed phenotype in HPV16/18-positive cervical cancer cells. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 20 , 6495–6503 (2014).
Ma, D. Safety Study of Zinc Finger Nucleases ZFN-602 and ZFN-758 in HPV-Infected Subjects . https://clinicaltrials.gov/study/NCT02800369 (2017).
Wang, J. et al. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res . 44 , e30 (2016).
Joung, J. K. & Sander, J. D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14 , 49–55 (2013).
Baker, M. Gene-editing nucleases. Nat. Methods 9 , 23–26 (2012).
Mock, U. et al. Novel lentiviral vectors with mutated reverse transcriptase for mRNA delivery of TALE nucleases. Sci. Rep. 4 , 6409 (2014).
Holkers, M. et al. Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic Acids Res. 41 , e63 (2013).
Qasim, W. et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9 , eaaj2013 (2017).
Ma, D. Safety Study of Transcription Activator-like Effector Nucleases T512 in HPV16-Infected Subjects . https://clinicaltrials.gov/study/NCT03226470 (2021).
She, X. et al. Immune surveillance of brain metastatic cancer cells is mediated by IFITM1. EMBO J. 42 , e111112 (2023).
Dong, M. B. et al. Systematic Immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell 178 , 1189–1204.e23 (2019).
Chiu, D. K.-C. et al. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology 159 , 609–623 (2020).
Chen, Z. et al. In vivo CD8+ T cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell 184 , 1262–1280.e22 (2021).
Hu, Y. et al. Safety and efficacy of CRISPR-based non-viral PD1 locus specifically integrated anti-CD19 CAR-T cells in patients with relapsed or refractory Non-Hodgkin’s lymphoma: a first-in-human phase I study. EClinicalMedicine 60 , 102010 (2023).
Shakiba, M. et al. TCR signal strength defines distinct mechanisms of T cell dysfunction and cancer evasion. J. Exp. Med. 219 , e20201966 (2022).
Zhang, Y. et al. Cyclosporine A-resistant CAR-T cells mediate antitumour immunity in the presence of allogeneic cells. Nat. Commun. 14 , 8491 (2023).
Tsujino, T. et al. CRISPR screens reveal genetic determinants of PARP inhibitor sensitivity and resistance in prostate cancer. Nat. Commun. 14 , 252 (2023).
Vojnic, M. et al. Acquired BRAF rearrangements induce secondary resistance to EGFR therapy in EGFR-mutated lung cancers. J. Thorac. Oncol. Publ. Int. Assoc. Study Lung Cancer 14 , 802–815 (2019).
Chow, R. D. et al. AAV-mediated direct in vivo CRISPR screen identifies functional suppressors in glioblastoma. Nat. Neurosci. 20 , 1329–1341 (2017).
Ye, L. et al. In vivo CRISPR screening in CD8 T cells with AAV–Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat. Biotechnol. 37 , 1302–1313 (2019).
Dervovic, D. et al. In vivo CRISPR screens reveal Serpinb9 and Adam2 as regulators of immune therapy response in lung cancer. Nat. Commun. 14 , 3150 (2023).
Lu, Y. et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 26 , 732–740 (2020).
Deng, L. et al. Identifying CDC7 as a synergistic target of chemotherapy in resistant small-cell lung cancer via CRISPR/Cas9 screening. Cell Death Discov. 9 , 1–10 (2023).
Wei, L. et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat. Commun. 10 , 4681 (2019).
Li, L. et al. In vitro CRISPR screening uncovers CRTC3 as a regulator of IFN-γ-induced ferroptosis of hepatocellular carcinoma. Cell Death Discov. 9 , 1–11 (2023).
Google Scholar
Benichou, E. et al. The transcription factor ChREBP Orchestrates liver carcinogenesis by coordinating the PI3K/AKT signaling and cancer metabolism. Nat. Commun. 15 , 1879 (2024).
Porto, E. M., Komor, A. C., Slaymaker, I. M. & Yeo, G. W. Base editing: advances and therapeutic opportunities. Nat. Rev. Drug Discov. 19 , 839–859 (2020).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533 , 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551 , 464–471 (2017).
Rees, H. A. et al. Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat. Commun. 8 , 15790 (2017).
Komor, A. C. et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 3 , eaao4774 (2017).
Webber, B. R. et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat. Commun. 10 , 5222 (2019).
Sayed, S. et al. Efficient correction of oncogenic KRAS and TP53 mutations through CRISPR base editing. Cancer Res 82 , 3002–3015 (2022).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576 , 149–157 (2019).
Chen, P. J. & Liu, D. R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 24 , 161–177 (2023).
Zhao, Z., Shang, P., Mohanraju, P. & Geijsen, N. Prime editing: advances and therapeutic applications. Trends Biotechnol. 41 , 1000–1012 (2023).
Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184 , 5635–5652.e29 (2021).
Fiumara, M. et al. Genotoxic effects of base and prime editing in human hematopoietic stem cells. Nat. Biotechnol . 1–15 https://doi.org/10.1038/s41587-023-01915-4 (2023).
Liu, P. et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat. Commun. 12 , 2121 (2021).
Davis, J. R. et al. Efficient prime editing in mouse brain, liver and heart with dual AAVs. Nat. Biotechnol. 42 , 253–264 (2024).
Abuhamad, A. Y. et al. Reverting TP53 Mutation in Breast Cancer Cells: Prime Editing Workflow and Technical Considerations. Cells 11 , 1612 (2022).
Jang, G., Kweon, J. & Kim, Y. CRISPR prime editing for unconstrained correction of oncogenic KRAS variants. Commun. Biol. 6 , 1–7 (2023).
Pomeroy, E. J. et al. Multiplex prime editing and PASSIGE TM for non-viral generation of an allogeneic CAR-T cell product. Blood 142 , 4803 (2023).
Zhou, L. & Yao, S. Recent advances in therapeutic CRISPR-Cas9 genome editing: mechanisms and applications. Mol. Biomed. 4 , 10 (2023).
Ahmadi, S. E. et al. Viral vectors and extracellular vesicles: Innate delivery systems utilized in CRISPR/Cas-mediated cancer therapy. Cancer Gene Ther. 30 , 936–954 (2023).
Münch, R. C. et al. Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors. Nat. Commun. 6 , 6246 (2015).
Gene Transfer Clinical Study in X-Linked Myotubular Myopathy - Full Text View - ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT03199469 .
Morrison, C. Fresh from the biotech pipeline—2019. Nat. Biotechnol. 38 , 126–131 (2020).
George, L. A. et al. Multiyear Factor VIII Expression after AAV gene transfer for Hemophilia A. N. Engl. J. Med. 385 , 1961–1973 (2021).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184 , 4919–4938.e22 (2021).
Liu, Y. et al. Cryo-electron tomography of NLRP3-activated ASC complexes reveals organelle co-localization. Nat. Commun. 14 , 7246 (2023).
Smith, C. C. et al. Alternative tumour-specific antigens. Nat. Rev. Cancer 19 , 465–478 (2019).
Tam, Y. Y. C., Chen, S. & Cullis, P. R. Advances in Lipid Nanoparticles for siRNA Delivery. Pharmaceutics 5 , 498–507 (2013).
Kafil, V. & Omidi, Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. BioImpacts BI 1 , 23–30 (2011).
Rosenblum, D. et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 6 , eabc9450 (2020).
Zhang, L. et al. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 9 , e441 (2017).
Li, C. et al. Ionizable lipid-assisted efficient hepatic delivery of gene editing elements for oncotherapy. Bioact. Mater. 9 , 590–601 (2022).
Luo, H. et al. Stress-release design for high-capacity and long-time lifespan aqueous zinc-ion batteries. Mater. Today Energy 21 , 100799 (2021).
Wu, Z. et al. CRISPR/Cas9-3NLS/sgHMGA2@PDA nanosystem is the potential efficient gene editing therapy for gastric cancer with HMGA2 high expression. Front. Oncol. 12 , 978533 (2022).
Yu, J. et al. Design of a self-driven probiotic-CRISPR/Cas9 nanosystem for sono-immunometabolic cancer therapy. Nat. Commun . 13 , 7903 (2022).
Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 12 , 31–46 (2022).
Chiari, R. et al. Two antigens recognized by autologous cytolytic T lymphocytes on a melanoma result from a single point mutation in an essential housekeeping gene. Cancer Res 59 , 5785–5792 (1999).
Wang, R.-F., Wang, X., Atwood, A. C., Topalian, S. L. & Rosenberg, S. A. Cloning genes encoding MHC Class II-restricted antigens: Mutated CDC27 as a tumor antigen. Science 284 , 1351–1354 (1999).
Perumal, D. et al. Mutation-derived Neoantigen-specific T-cell responses in multiple myeloma. Clin. Cancer Res. 26 , 450–464 (2020).
Turajlic, S. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18 , 1009–1021 (2017).
Mitelman, F., Johansson, B. & Mertens, F. Fusion genes and rearranged genes as a linear function of chromosome aberrations in cancer. Nat. Genet. 36 , 331–334 (2004).
Eckhardt, M. et al. Multiple Routes To Oncogenesis are promoted by the human Papillomavirus–Host protein network. Cancer Discov. 8 , 1474–1489 (2018).
HEPAVAC Consortium. et al. Multi-omics discovery of exome-derived neoantigens in hepatocellular carcinoma. Genome Med 11 , 28 (2019).
De Mattos-Arruda, L. et al. Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 31 , 978–990 (2020).
O’Donnell, T. J. et al. MHCflurry: Open-source Class I MHC binding affinity prediction. Cell Syst. 7 , 129–132.e4 (2018).
Wells, D. K. et al. Key parameters of tumor Epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183 , 818–834.e13 (2020).
Roudko, V. et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell 183 , 1634–1649.e17 (2020).
Tanyi, J. L. et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 10 , eaao5931 (2018).
Schoen, R. E. et al. Randomized, double-blind, placebo-controlled trial of MUC1 peptide vaccine for prevention of recurrent Colorectal Adenoma. Clin. Cancer Res. 29 , 1678–1688 (2023).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547 , 217–221 (2017).
Hubbard, J. M. et al. Safety and activity of PolyPEPI1018 combined with maintenance therapy in metastatic colorectal cancer: an open-label, multicenter, Phase Ib Study. Clin. Cancer Res. 28 , 2818–2829 (2022).
Hu, Z. et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 27 , 515–525 (2021).
Schuhmacher, J. et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: results from a phase I/II clinical trial. J. Immunother. Cancer 8 , e001157 (2020).
Obara, W. et al. A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder. Ann. Oncol. 28 , 798–803 (2017).
Lynn, G. M. et al. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 38 , 320–332 (2020).
Ni, Q. et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 6 , eaaw6071 (2020).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547 , 222–226 (2017).
Zhang, H. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl Acad. Sci. 118 , e2005191118 (2021).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618 , 144–150 (2023).
Cafri, G. et al. mRNA vaccine–induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 130 , 5976–5988 (2020).
Wang, Z. et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 11 , 711–721 (2004).
Tiriveedhi, V. et al. Safety and preliminary evidence of biologic efficacy of a mammaglobin-A DNA vaccine in patients with stable metastatic breast cancer. Clin. Cancer Res. 20 , 5964–5975 (2014).
Cecil, D. L. et al. Immunization with a Plasmid DNA vaccine encoding the N-Terminus of insulin-like growth factor binding Protein-2 in advanced ovarian cancer leads to high-level Type I immune responses. Clin. Cancer Res. 27 , 6405–6412 (2021).
Trimble, C. L. et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386 , 2078–2088 (2015).
Steinman, R. M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30 , 1–22 (2012).
Carreno, B. M. et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348 , 803–808 (2015).
Liau, L. M. et al. Association of autologous Tumor Lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent Glioblastoma: A Phase 3 prospective externally controlled cohort trial. JAMA Oncol. 9 , 112 (2023).
Mahoney, D. J., Stojdl, D. F. & Laird, G. Virus therapy for cancer. Sci. Am. 311 , 54–59 (2014).
Anderson, B. D., Nakamura, T., Russell, S. J. & Peng, K.-W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles Virus. Cancer Res 64 , 4919–4926 (2004).
Jayawardena, N., Burga, L. N., Poirier, J. T. & Bostina, M. Virus–receptor interactions: structural insights for oncolytic virus development. Oncol. Virother. 8 , 39–56 (2019).
Gopinath, S. C. B. et al. Analysis of compounds that interfere with Herpes Simplex Virus–host receptor interactions using surface plasmon resonance. Anal. Chem. 85 , 10455–10462 (2013).
Yu, Z. et al. Enhanced Nectin-1 Expression and Herpes oncolytic sensitivity in highly migratory and invasive carcinoma. Clin. Cancer Res. 11 , 4889–4897 (2005).
Karki, R. et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 7 , eabo6294 (2022).
Gozgit, J. M. et al. PARP7 negatively regulates the type I interferon response in cancer cells and its inhibition triggers antitumor immunity. Cancer Cell 39 , 1214–1226.e10 (2021).
Flores, E. B., Aksoy, B. A. & Bartee, E. Initial dose of oncolytic myxoma virus programs durable antitumor immunity independent of in vivo viral replication. J. Immunother. Cancer 8 , e000804 (2020).
Ma, J. et al. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 11 , 48 (2020).
Ye, K. et al. An armed oncolytic virus enhances the efficacy of tumor-infiltrating lymphocyte therapy by converting tumors to artificial antigen-presenting cells in situ. Mol. Ther. 30 , 3658–3676 (2022).
Bommareddy, P. K., Aspromonte, S., Zloza, A., Rabkin, S. D. & Kaufman, H. L. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 10 , eaau0417 (2018).
Ramelyte, E. et al. Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell 39 , 394–406.e4 (2021).
Andtbacka, R. H. I. et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33 , 2780–2788 (2015).
Ott, P. A. & Hodi, F. S. Talimogene Laherparepvec for the treatment of advanced melanoma. Clin. Cancer Res. 22 , 3127–3131 (2016).
Soliman, H. et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat. Med. 29 , 450–457 (2023).
Harrington, K. J. et al. Talimogene Laherparepvec and Pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): A multicenter, Phase 1b study. Clin. Cancer Res. 26 , 5153–5161 (2020).
Kelly, C. M. et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with Pembrolizumab: A Phase 2 clinical trial. JAMA Oncol. 6 , 402 (2020).
Fueyo, J. et al. Preclinical characterization of the antiglioma activity of a Tropism-Enhanced Adenovirus Targeted to the Retinoblastoma Pathway. JNCI J. Natl Cancer Inst. 95 , 652–660 (2003).
Lang, F. F. et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and immunotherapeutic effects in recurrent malignant Glioma. J. Clin. Oncol. 36 , 1419–1427 (2018).
Nassiri, F. et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial. Nat. Med. 29 , 1370–1378 (2023).
Todo, T., Martuza, R. L., Rabkin, S. D. & Johnson, P. A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl Acad. Sci. 98 , 6396–6401 (2001).
Todo, T., Ino, Y., Ohtsu, H., Shibahara, J. & Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 13 , 4119 (2022).
Curti, B. D. et al. Intratumoral oncolytic virus V937 plus ipilimumab in patients with advanced melanoma: the phase 1b MITCI study. J. Immunother. Cancer 10 , e005224 (2022).
Mahalingam, D. et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. 26 , 71–81 (2020).
Monge, C. et al. Phase I/II study of PexaVec in combination with immune checkpoint inhibition in refractory metastatic colorectal cancer. J. Immunother. Cancer 11 , e005640 (2023).
Chesney, J. A. et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 11 , e006270 (2023).
Schenk, E. L. et al. A randomized double-blind Phase II Study of the Seneca Valley Virus (NTX-010) versus Placebo for patients with extensive-stage SCLC (ES SCLC) who were stable or responding after at least four cycles of Platinum-based chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J. Thorac. Oncol. 15 , 110–119 (2020).
Ponce, S. et al. ONCOS-102 plus pemetrexed and platinum chemotherapy in malignant pleural mesothelioma: a randomized phase 2 study investigating clinical outcomes and the tumor microenvironment. J. Immunother. Cancer 11 , e007552 (2023).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19 , 329–336 (2013).
Cavenee, W. K. et al. Genetic origin of mutations predisposing to Retinoblastoma. Science 228 , 501–503 (1985).
Xiao, B. et al. Crystal structure of the retinoblastoma tumor suppressor protein bound to E2F and the molecular basis of its regulation. Proc. Natl Acad. Sci. 100 , 2363–2368 (2003).
Pascual-Pasto, G. et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci. Transl. Med. 11 , eaat9321 (2019).
Hwang, T.-H. et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic Poxvirus, in patients with metastatic melanoma. Mol. Ther. 19 , 1913–1922 (2011).
Zhang, B. et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J. Immunother. Cancer 9 , e002224 (2021).
Annels, N. E. et al. Phase I Trial of an ICAM-1-Targeted Immunotherapeutic-Coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin. Cancer Res. 25 , 5818–5831 (2019).
Fares, J. et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 22 , 1103–1114 (2021).
Ruano, D. et al. First-in-human, first-in-child trial of autologous MSCs carrying the oncolytic Virus Icovir-5 in patients with advanced tumors. Mol. Ther. 28 , 1033–1042 (2020).
Chow, A., Perica, K., Klebanoff, C. A. & Wolchok, J. D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 19 , 775–790 (2022).
Coley, W. B. The treatment of inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R. Soc. Med. 3 , 1–48 (1910).
Brunschwig, A. The efficacy Of “Coleyʼs Toxin” in the treatment of Sarcoma * An experimental study. Ann. Surg. 109 , 109–113 (1939).
Karbach, J. et al. Phase I clinical trial of mixed bacterial vaccine (Coley’s Toxins) in patients with NY-ESO-1 Expressing cancers: immunological effects and clinical activity. Clin. Cancer Res. 18 , 5449–5459 (2012).
Babjuk, M. et al. EAU Guidelines on Non–Muscle-Invasive Urothelial Carcinoma of the Bladder, the 2011 Update. Eur. Urol. 59 , 997–1008 (2011).
Bisiaux, A. et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J. Urol. 181 , 1571–1580 (2009).
Brandau, S. et al. NK cells are essential for effective BCG immunotherapy. Int. J. Cancer 92 , 697–702 (2001).
Stefanini, G. F. et al. Class I and Class II HLA antigen expression by transitional cell carcinoma of the bladder: correlation with T-cell infiltration and BCG treatment. J. Urol. 141 , 1449–1453 (1989).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34 , 637–650 (2011).
Aleynick, M., Svensson-Arvelund, J., Flowers, C. R., Marabelle, A. & Brody, J. D. Pathogen molecular pattern receptor agonists: treating cancer by mimicking infection. Clin. Cancer Res. 25 , 6283–6294 (2019).
Nooka, A. K. et al. Assessment of safety and immunogenicity of PVX-410 vaccine with or without lenalidomide in patients with smoldering multiple myeloma: a nonrandomized clinical trial. JAMA Oncol. 4 , e183267 (2018).
Hammerich, L. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25 , 814–824 (2019).
Melssen, M. M. et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J. Immunother. Cancer 7 , 163 (2019).
Bhatia, S. et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin. Cancer Res. 25 , 1185–1195 (2019).
Ferris, R. L. et al. Effect of adding Motolimod to Standard combination Chemotherapy and Cetuximab treatment of patients with squamous cell carcinoma of the head and neck: the Active8 randomized clinical trial. JAMA Oncol. 4 , 1583 (2018).
Kjellberg, R. N., Sweet, W. H., Preston, W. M. & Koehler, A. M. The Bragg peak of a proton beam in intracranial therapy of tumors. Trans. Am. Neurol. Assoc. 87 , 216–218 (1962).
Lomax, A. J. et al. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother. Oncol. 51 , 257–271 (1999).
Halperin, E. C. Particle therapy and treatment of cancer. Lancet Oncol. 7 , 676–685 (2006).
Slater, J. M. et al. The proton treatment center at Loma Linda University Medical Center: Rationale for and description of its development. Int. J. Radiat. Oncol. 22 , 383–389 (1992).
Schulz-Ertner, D. & Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 25 , 953–964 (2007).
Eekers, D. B. P. et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiother. Oncol. 121 , 387–394 (2016).
Waddle, M. R. et al. Survival after proton and photon radiation therapy in patients with head and neck cancers: A study of the national cancer database. Int. J. Radiat. Oncol. 100 , 1330 (2018).
Lee, A. et al. Evaluation of Proton therapy reirradiation for patients with recurrent head and neck squamous cell carcinoma. JAMA Netw. Open 6 , e2250607 (2023).
Mizoe, J. et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother. Oncol. 103 , 32–37 (2012).
Schulz-Ertner, D. et al. Results of carbon ion radiotherapy in 152 patients. Int. J. Radiat. Oncol. 58 , 631–640 (2004).
Chang, J. Y. et al. Proton Beam Radiotherapy and concurrent chemotherapy for unresectable Stage III non–small cell lung cancer: final results of a Phase 2 study. JAMA Oncol. 3 , e172032 (2017).
Gjyshi, O. et al. Toxicity and survival after intensity-modulated proton therapy versus passive scattering proton therapy for NSCLC. J. Thorac. Oncol. 16 , 269–277 (2021).
Liao, Z. et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non–small-cell lung cancer. J. Clin. Oncol. 36 , 1813–1822 (2018).
Karube, M. et al. Single-fraction carbon-ion radiation therapy for patients 80 years of age and older with Stage I non-small cell lung cancer. Int. J. Radiat. Oncol. 95 , 542–548 (2016).
Yamamoto, N. et al. A dose escalation clinical trial of single-fraction carbon ion radiotherapy for peripheral Stage I non–small cell lung cancer. J. Thorac. Oncol. 12 , 673–680 (2017).
Goldin, G. H. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307 , 1611 (2012).
Moteabbed, M. et al. A prospective comparison of the effects of interfractional variations on proton therapy and intensity modulated radiation therapy for prostate cancer. Int. J. Radiat. Oncol. 95 , 444–453 (2016).
Eichkorn, T. et al. Results of a prospective randomized trial on long-term effectiveness of protons and carbon ions in prostate cancer: LEM I and α/β = 2 Gy overestimates the RBE. Radiother. Oncol. 173 , 223–230 (2022).
Nomiya, T. et al. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: A report from the Japan Carbon ion Radiation Oncology Study Group (J-CROS). Radiother. Oncol. 121 , 288–293 (2016).
Ackroyd, R., Kelty, C., Brown, N. & Reed, M. The history of Photodetection and Photodynamic therapy. Photochem. Photobiol. 74 , 656 (2001).
Pérez-Hernández, M. et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold Nanoprisms. ACS Nano 9 , 52–61 (2015).
Yamamoto, N., Homma, S., Sery, T. W., Donoso, L. A. & Kenneth Hoober, J. Photodynamic immunopotentiation: in vitro activation of macrophages by treatment of mouse peritoneal cells with haematoporphyrin derivative and light. Eur. J. Cancer Clin. Oncol. 27 , 467–471 (1991).
Agarwal, M. L., Larkin, H. E., Zaidi, S. I., Mukhtar, H. & Oleinick, N. L. Phospholipase activation triggers apoptosis in photosensitized mouse lymphoma cells. Cancer Res. 53 , 5897–5902 (1993).
Gollnick, S. O., Vaughan, L. & Henderson, B. W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res 62 , 1604–1608 (2002).
Rastinehad, A. R. et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl Acad. Sci. 116 , 18590–18596 (2019).
Fisher, C. et al. Photodynamic therapy for the treatment of vertebral metastases: A Phase I clinical trial. Clin. Cancer Res. 25 , 5766–5776 (2019).
Chen, B. & De Witte, P. A. Photodynamic therapy efficacy and tissue distribution of hypericin in a mouse P388 lymphoma tumor model. Cancer Lett. 150 , 111–117 (2000).
Kim, E. J. et al. Efficacy and safety of topical Hypericin Photodynamic Therapy for early-stage cutaneous T-cell Lymphoma (Mycosis Fungoides): The FLASH Phase 3 randomized clinical trial. JAMA Dermatol. 158 , 1031 (2022).
Folkman, J. Angiogenesis. Annu. Rev. Med. 57 , 1–18 (2006).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146 , 873–887 (2011).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176 , 1248–1264 (2019).
Dvorak, H. F. How tumors make bad blood vessels and stroma. Am. J. Pathol. 162 , 1747–1757 (2003).
Ruch, C., Skiniotis, G., Steinmetz, M. O., Walz, T. & Ballmer-Hofer, K. Structure of a VEGF–VEGF receptor complex determined by electron microscopy. Nat. Struct. Mol. Biol. 14 , 249–250 (2007).
Kawasaki, K. et al. Ras signaling directs endothelial specification of VEGFR2+ vascular progenitor cells. J. Cell Biol. 181 , 131–141 (2008).
Takahashi, T., Ueno, H. & Shibuya, M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18 , 2221–2230 (1999).
Ruan, G.-X. & Kazlauskas, A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt: VEGF-dependent activation of PI3K/Akt requires Axl. EMBO J. 31 , 1692–1703 (2012).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2 , 1096–1103 (1996).
Terme, M. et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73 , 539–549 (2013).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212 , 139–148 (2015).
Sini, P. et al. The antitumor and antiangiogenic activity of vascular endothelial growth factor receptor inhibition is potentiated by ErbB1 blockade. Clin. Cancer Res. 11 , 4521–4532 (2005).
Fons, P. et al. Tumor vasculature is regulated by FGF/FGFR signaling‐mediated angiogenesis and bone marrow‐derived cell recruitment: this mechanism is inhibited by SSR128129E, the first allosteric antagonist of FGFRs. J. Cell. Physiol. 230 , 43–51 (2015).
Hurwitz, H. et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 350 , 2335–2342 (2004).
Ray-Coquard, I. et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 381 , 2416–2428 (2019).
Wick, W. et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 377 , 1954–1963 (2017).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24 , 749–757 (2018).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15 , 1224–1235 (2014).
Garon, E. B. et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384 , 665–673 (2014).
Tabernero, J. et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 16 , 499–508 (2015).
Zhu, A. X. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16 , 859–870 (2015).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356 , 125–134 (2007).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359 , 378–390 (2008).
Brose, M. S. et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384 , 319–328 (2014).
Li, J. et al. Randomized, double-blind, placebo-controlled Phase III trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic Adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34 , 1448–1454 (2016).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391 , 1163–1173 (2018).
Motzer, R. J. et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295 , 2516 (2006).
Reck, M. et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 15 , 143–155 (2014).
Zhang, X. et al. Efficacy and Safety of Apatinib Plus Vinorelbine in patients with wild-type advanced non–small cell lung cancer after second-line treatment failure: a nonrandomized clinical trial. JAMA Netw. Open 3 , e201226 (2020).
Lu, S. et al. Randomized, double-blind, placebo-controlled, multicenter Phase II Study of Fruquintinib after two prior Chemotherapy Regimens in Chinese patients with advanced nonsquamous non‒small-cell lung cancer. J. Clin. Oncol. 36 , 1207–1217 (2018).
Shackelford, D. B. & Shaw, R. J. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9 , 563–575 (2009).
Bonanno, L. et al. LKB1 Expression correlates with increased survival in patients with advanced non–small cell lung cancer treated with chemotherapy and Bevacizumab. Clin. Cancer Res. 23 , 3316–3324 (2017).
Cascone, T. et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor–resistant human lung adenocarcinoma. J. Clin. Invest. 121 , 1313–1328 (2011).
He, H., Liu, L., Morin, E. E., Liu, M. & Schwendeman, A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc. Chem. Res. 52 , 2445–2461 (2019).
Zhang, Z. et al. Functionalization and higher-order organization of liposomes with DNA nanostructures. Nat. Commun. 14 , 5256 (2023).
Wang, W. et al. Resveratrol-Loaded TPGS-Resveratrol-solid lipid nanoparticles for multidrug-resistant therapy of breast cancer: in vivo and in vitro study. Front. Bioeng. Biotechnol. 9 , 762489 (2021).
Zheng, G., Zheng, M., Yang, B., Fu, H. & Li, Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: Synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed. Pharmacother. Biomed. Pharmacother. 116 , 109006 (2019).
Rayamajhi, S., Nguyen, T. D. T., Marasini, R. & Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 94 , 482–494 (2019).
Basti, H. et al. Catechol derivatives-coated Fe3O4 and gamma-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci. 341 , 248–254 (2010).
Hwang, C., Choi, M.-H., Kim, H.-E., Jeong, S.-H. & Park, J.-U. Reactive oxygen species-generating hydrogel platform for enhanced antibacterial therapy. NPG Asia Mater. 14 , 1–15 (2022).
Wang, Y., Zhang, Q., Wang, B., Li, P. & Liu, P. LiCl treatment induces programmed cell death of Schwannoma cells through AKT- and MTOR-mediated Necroptosis. Neurochem. Res. 42 , 2363–2371 (2017).
Hou, L. et al. Hybrid-membrane-decorated Prussian blue for effective cancer immunotherapy via tumor-associated macrophages polarization and Hypoxia relief. Adv. Mater. Deerfield Beach Fla 34 , e2200389 (2022).
Wang, N., Liang, H. & Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 5 , 614 (2014).
Chaudhari, D. et al. Exploring paclitaxel-loaded adenosine-conjugated PEGylated PLGA nanoparticles for targeting triple-negative breast cancer. Drug Deliv. Transl. Res. 13 , 1074–1087 (2023).
Lee, C. K. et al. Anti-PD-L1 F(ab) conjugated PEG-PLGA nanoparticle enhances immune checkpoint therapy. Nanotheranostics 6 , 243–255 (2022).
Chang, C.-S. et al. Anti-cancer effect of fenbendazole-incorporated PLGA nanoparticles in ovarian cancer. J. Gynecol. Oncol. 34 , e58 (2023).
Cruz-Nova, P. et al. Chemo-radiotherapy with 177Lu-PLGA(RGF)-CXCR4L for the targeted treatment of colorectal cancer. Front. Med. 10 , 1191315 (2023).
Kim, G. T. et al. PLAG co-treatment increases the anticancer effect of Adriamycin and cyclophosphamide in a triple-negative breast cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 619 , 110–116 (2022).
Dubey, S. K. et al. Recent advances of dendrimers as multifunctional nano-carriers to combat breast cancer. Eur. J. Pharm. Sci. 164 , 105890 (2021).
Huynh, K.-H. et al. Bioapplications of Nanomaterials. in Nanotechnology for Bioapplications (ed. Jun, B.-H.) 235–255 (Springer, Singapore). https://doi.org/10.1007/978-981-33-6158-4_10 (2021).
Novartis Tafinlar + Mekinist approved by FDA for pediatric patients with BRAF V600E low-grade glioma, the most common pediatric brain cancer. Novartis https://www.novartis.com/news/media-releases/novartis-tafinlar-mekinist-approved-fda-pediatric-patients-braf-v600e-low-grade-glioma-most-common-pediatric-brain-cancer .
Shah, M. A. et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat. Med. 29 , 2133–2141 (2023).
FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate (2023).
Mai, H.-Q. et al. Toripalimab Plus chemotherapy for recurrent or metastatic nasopharyngeal Carcinoma: The JUPITER-02 randomized clinical trial. JAMA 330 , 1961–1970 (2023).
Planchard, D. et al. Osimertinib with or without Chemotherapy in EGFR-mutated advanced NSCLC. N. Engl. J. Med. 389 , 1935–1948 (2023).
Colombo, Nicoletta et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N. Engl. J. Med. 385 , 1856–1867 (2021).
Motzer Robert, J. et al. Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378 , 1277–1290 (2018).
Rini Brian, I. et al. Pembrolizumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380 , 1116–1127 (2019).
Motzer Robert, J. et al. Avelumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380 , 1103–1115 (2019).
Choueiri Toni, K. et al. Nivolumab plus Cabozantinib versus Sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384 , 829–841 (2021).
Albiges, L. et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 24 , 881–891 (2023).
Tawbi Hussein, A. et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 386 , 24–34 (2022).
Zhang, G. et al. GD2 CAR-T cells in combination with Nivolumab exhibit enhanced antitumor efficacy. Transl. Oncol. 32 , 101663 (2023).
Gong, L. et al. Nasopharyngeal carcinoma cells promote regulatory T cell development and suppressive activity via CD70-CD27 interaction. Nat. Commun . 14 , 1912 (2023).
Hashimoto, M. et al. PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program. Nature 610 , 173–181 (2022).
Cataldo Vince, D., Gibbons Don, L., Pérez-Soler, R. & Quintás-Cardama, A. Treatment of non–small-cell lung cancer with Erlotinib or Gefitinib. N. Engl. J. Med. 364 , 947–955 (2011).
Jóri, B. et al. The combined therapy of Cabozantinib, Crizotinib, and Osimertinib in a lung cancer patient with acquired MET amplification and resistance mutations. Curr. Oncol. 30 , 8805–8814 (2023).
von Minckwitz, G. et al. Adjuvant Pertuzumab and Trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377 , 122–131 (2017).
Spriggs David, R. & Longo Dan, L. PARP inhibitors in ovarian cancer treatment. N. Engl. J. Med. 375 , 2197–2198 (2016).
Turner Nicholas, C. et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N. Engl. J. Med. 373 , 209–219 (2015).
Montesinos, Pau et al. Ivosidenib and Azacitidine in IDH1-mutated acute myeloid leukemia. N. Engl. J. Med. 386 , 1519–1531 (2022).
Liu, B. et al. Targeting TROY-mediated P85a/AKT/TBX3 signaling attenuates tumor stemness and elevates treatment response in hepatocellular carcinoma. J. Exp. Clin. Cancer Res . 41 , 1–19 (2022).
Jiang, Y.-Y. et al. TP63, SOX 2 , and KLF5 establish a core regulatory circuitry that controls epigenetic and transcription patterns in esophageal squamous cell carcinoma cell lines. Gastroenterology 159 , 1311–1327.e19 (2020).
Zheng, C. et al. Donafenib and GSK-J4 synergistically induce ferroptosis in liver cancer by upregulating HMOX1 Expression. Adv. Sci. Weinh. Baden.-Wurtt. Ger. 10 , e2206798 (2023).
Xu, Z. et al. Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 7 , e013053 (2017).
Yaeger, R. et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med . 388 , 44–54 (2023).
Novartis Pharmaceuticals. A Randomised, Multi-Centre, Open-Label, Phase III Study of Adjuvant Lapatinib, Trastuzumab, Their Sequence and Their Combination in Patients With HER2/ErbB2 Positive Primary Breast Cancer . https://clinicaltrials.gov/study/NCT00490139 (2021).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378 , 2288–2301 (2018).
Loupakis, F. et al. Initial Therapy with FOLFOXIRI and Bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 371 , 1609–1618 (2014).
Hosaka, K. et al. KRAS mutation-driven angiopoietin 2 bestows anti-VEGF resistance in epithelial carcinomas. Proc. Natl Acad. Sci. USA 120 , e2303740120 (2023).
Srinivasan, R. et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J. Clin. Oncol. 38 , 5004 (2020). 5004.
Prager, G. W. et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med . 388 , 1657–1667 (2023).
Friedlander, M. et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 20 , 1306–1315 (2019).
Kanjee, Z., Crowe, B. & Rodman, A. Accuracy of a Generative Artificial Intelligence Model in a Complex Diagnostic Challenge. JAMA 330 , 78–80 (2023).
Liberti, M. V. & Locasale, J. W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 41 , 211 (2016).
Xu, D. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 580 , 530–535 (2020).
Chen, J., Li, G., Sun, D., Li, H. & Chen, L. Research progress of hexokinase 2 in inflammatory-related diseases and its inhibitors. Eur. J. Med. Chem. 264 , 115986 (2024).
Fu, K. et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 187 , 882–896.e17 (2024).
Ling, A. L. et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 623 , 157–166 (2023).
Cui, C. et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC–IV melanoma: a phase Ib study. J. Immunother. Cancer 10 , e004307 (2022).
Gállego Pérez-Larraya, J. et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic Pontine Glioma. N. Engl. J. Med. 386 , 2471–2481 (2022).
Bazan-Peregrino, M. et al. VCN-01 disrupts pancreatic cancer stroma and exerts antitumor effects. J. Immunother. Cancer 9 , e003254 (2021).
Heo, J. et al. Safety and dose escalation of the targeted oncolytic adenovirus OBP-301 for refractory advanced liver cancer: Phase I clinical trial. Mol. Ther. 31 , 2077–2088 (2023).
Shirakawa, Y. et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 153 , 98–108 (2021).
Nemunaitis, J. et al. A Phase I Study of Telomerase-specific Replication Competent Oncolytic Adenovirus (Telomelysin) for various solid tumors. Mol. Ther. 18 , 429–434 (2010).
Fakih, M. et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer: a phase I clinical trial (SPICE). J. Immunother. Cancer 11 , e006561 (2023).
Garcia-Carbonero, R. et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 5 , 71 (2017).
Machiels, J.-P. et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J. Immunother. Cancer 7 , 20 (2019).
Dispenzieri, A. et al. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia 31 , 2791–2798 (2017).
Packiriswamy, N. et al. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 34 , 3310–3322 (2020).
Galanis, E. et al. Carcinoembryonic antigen-expressing oncolytic measles virus derivative in recurrent glioblastoma: a phase 1 trial. Nat. Commun. 15 , 493 (2024).
Galanis, E. et al. Phase I Trial of Intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 70 , 875–882 (2010).
Andtbacka, R. H. I. et al. Clinical responses of Oncolytic Coxsackievirus A21 (V937) in patients with unresectable melanoma. J. Clin. Oncol. 39 , 3829–3838 (2021).
Rudin, C. M. et al. Phase 1, open-label, dose-escalation study on the safety, pharmacokinetics, and preliminary efficacy of intravenous Coxsackievirus A21 (V937), with or without pembrolizumab, in patients with advanced solid tumors. J. Immunother. Cancer 11 , e005007 (2023).
Geletneky, K. et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 12 , 99 (2012).
Hajda, J. et al. Phase 2 trial of oncolytic H-1 Parvovirus therapy shows safety and signs of immune system activation in patients with metastatic pancreatic ductal Adenocarcinoma. Clin. Cancer Res. 27 , 5546–5556 (2021).
Hajda, J. et al. A non-controlled, single arm, open label, phase II study of intravenous and intratumoral administration of ParvOryx in patients with metastatic, inoperable pancreatic cancer: ParvOryx02 protocol. BMC Cancer 17 , 576 (2017).
Desjardins, A. et al. Recurrent Glioblastoma treated with recombinant Poliovirus. N. Engl. J. Med. 379 , 150–161 (2018).
Beasley, G. M. et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 9 , e002203 (2021).
Beasley, G. M. et al. Multimodality analysis confers a prognostic benefit of a T-cell infiltrated tumor microenvironment and peripheral immune status in patients with melanoma. J. Immunother. Cancer 10 , e005052 (2022).
Comins, C. et al. REO-10: A Phase I study of intravenous Reovirus and Docetaxel in patients with advanced cancer. Clin. Cancer Res. 16 , 5564–5572 (2010).
Galanis, E. et al. Phase II trial of intravenous administration of Reolysin® (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol. Ther. 20 , 1998–2003 (2012).
Noonan, A. M. et al. Randomized Phase 2 trial of the oncolytic Virus Pelareorep (Reolysin) in upfront treatment of metastatic pancreatic Adenocarcinoma. Mol. Ther. 24 , 1150–1158 (2016).
Sborov, D. W. et al. A Phase I trial of single-agent Reolysin in patients with relapsed multiple myeloma. Clin. Cancer Res. 20 , 5946–5955 (2014).
Jiffry, J. et al. Oncolytic Reovirus (pelareorep) induces autophagy in KRAS-mutated colorectal cancer. Clin. Cancer Res. 27 , 865–876 (2021).
Burke, M. J. et al. Phase I trial of Seneca Valley Virus (NTX‐010) in children with relapsed/refractory solid tumors: A report of the Children’s Oncology Group. Pediatr. Blood Cancer 62 , 743–750 (2015).
Toulmonde, M. et al. Randomized phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced soft-tissue sarcoma. J. Hematol. Oncol. J. Hematol. Oncol. 15 , 149 (2022).
Toulmonde, M. et al. Reshaping the tumor microenvironment of cold soft-tissue sarcomas with oncolytic viral therapy: a phase 2 trial of intratumoral JX-594 combined with avelumab and low-dose cyclophosphamide. Mol. Cancer 23 , 38 (2024).
Download references
Acknowledgements
This study was supported by a research grant from the Shenzhen Science and Technology Program (ZDSYS20210623091811035).
Author information
These authors contributed equally: Beilei Liu, Hongyu Zhou, Licheng Tan.
Authors and Affiliations
Department of Clinical Oncology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
Beilei Liu & Xin-Yuan Guan
Department of Clinical Oncology, The University of Hong Kong, Hong Kong, China
Beilei Liu, Hongyu Zhou, Licheng Tan, Kin To Hugo Siu & Xin-Yuan Guan
State Key Laboratory for Liver Research, The University of Hong Kong, Hong Kong, China
Advanced Energy Science and Technology Guangdong Laboratory, Huizhou, China
Xin-Yuan Guan
MOE Key Laboratory of Tumor Molecular Biology, Jinan University, Guangzhou, China
You can also search for this author in PubMed Google Scholar
Contributions
B.L.L., H.Y.Z., L.C.T., and S.K.T.H. wrote the manuscript and made the figures, B.L.L., H.Y.Z., L.C.T., organized the tables, X.Y.G. conceived, designed, and edited the manuscript. All authors have read and approved the article.
Corresponding author
Correspondence to Xin-Yuan Guan .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Supplementary information
Supplementary_materials, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Liu, B., Zhou, H., Tan, L. et al. Exploring treatment options in cancer: tumor treatment strategies. Sig Transduct Target Ther 9 , 175 (2024). https://doi.org/10.1038/s41392-024-01856-7
Download citation
Received : 23 January 2024
Revised : 24 April 2024
Accepted : 29 April 2024
Published : 17 July 2024
DOI : https://doi.org/10.1038/s41392-024-01856-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Trogocytosis in car immune cell therapy: a key mechanism of tumor immune escape.
- Yizhao Chen
- Qianling Xin
Cell Communication and Signaling (2024)
Metformin boosts doxorubicin efficacy and increases CD8 + T cell frequency in mouse breast cancer
- Elaheh Hassani
- Sahand Mozzendizaji
- Adel Mohammadzadeh
Clinical and Translational Oncology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
The Current Status of Gene Therapy for the Treatment of Cancer
Tafere mulaw belete.
- Author information
- Article notes
- Copyright and License information
Correspondence: Tafere Mulaw Belete P.o.box 196, Phone: Tel +251 9 18 04 59 43 Email [email protected]
Received 2021 Jan 22; Accepted 2021 Mar 4; Collection date 2021.
This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License ( http://creativecommons.org/licenses/by-nc/3.0/ ). By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms ( https://www.dovepress.com/terms.php ).
Gene therapy is the administration of foreign genomic material into the host tissue to modify the expression of a gene product or to change the biological properties of cells for therapeutic use. Initially, the major objective of gene therapy was to manage genetic diseases, but now different disorders with several patterns of acquired and inherited disorders are targets of gene therapy. Over three decades, the advancement of Genome engineering technologies facilitated gene therapy for the prevention and management of intractable diseases. Researchers are advancing with cautious optimism that safe and effective treatment will give to patients with single-gene disorders and complex acquired disorders. To date, over 3000 genes associates with disease-causing mutations, and about 2600 gene therapy trials are undergoing for the management of various disorders. This review summarizes the principles of genome-editing approaches, such as zinc finger nucleases, transcription activator-like effector nucleases, meganucleases, and the CRISPR/Cas9 system with the underlying mechanisms. This review also explains the types of gene delivery systems as viral [adenoviral, adeno association, herpes simplex virus] and nonviral delivery systems (physical: DNA bombardment, electroporation) and (chemical: Cationic lipids, cationic polymers). Finally, this review summarizes gene therapy medicines approved to treat cancer in detail, including names, indications, vectors, and mode of gene therapy. Gene therapy becomes an alternative to an existing management for different diseases. Therefore, gene products with safe vectors and better biotechnologies play a significant role in the prophylaxis and management of various disorders in the future.
Keywords: gene therapy, gene product, gene delivery, cancer, clinical trial
After DNA helical structure discovery, the world continuous staircase outburst of several advanced technologies, which are currently heading toward translation into clinical practice. Over the last decades, several molecular techniques developed that help to edit the DNA codes and modify mRNA by post-transcriptional modifications. Gene therapy is the delivery of specific genetic material to modify the encoding of a gene product or to change the biological properties of tissues for the management of various disorders. 1 Gene therapy overcomes the limitations associated with the recombinant therapeutic use of peptides, such as low bioavailability, instability, severe toxicity, clearance rates, and high production cost. 2 Gene therapies act by different mechanisms including, replacing malfunction genes with the therapeutic genes, gene knockdown, or deactivating problem genes, and insert a new gene to treat a disease. 3 Gene therapy can be done in either somatic or germline cells. In somatic cells, gene therapy only the modified tissues will be affected, but in germline cell gene therapy, genetic changes transmit to the offspring. So, there is no clinical trial on human germline gene therapy. 4 Currently, somatic gene therapy is safe for the management of several disorders in human beings. Gene therapy effectively treats several diseases due to increased understanding of disease pathogenesis and improved gene delivery technologies. 5 Gene therapy uses genetic material (ie, RNA or DNA) via a vector that facilitates the delivery of foreign genetic material into the host organ. The genetic material is administered into the target organ (in vivo gene therapy) or used to modify cells taken from the host that are then re-administered (ex vivo gene therapy). Gene therapy aims to provide a functional gene copy of the damaged gene(s), increase the availability of disease-modifying genes or suppress the activity of a damaged gene. 6 , 7 Gene therapy has a broad spectrum of applications, from gene replacement and knockdown for genetic disorders including cancer, hemophilia, hypercholesterolemia, and neurodegenerative diseases to vaccination, each with different requirements for gene administration. 8 Gene delivery systems consist of three components: a gene that expresses essential therapeutic peptides, a plasmid-based gene encoding system that regulates the activity of a gene in the target organ, and a gene delivery system that regulates the administration of the encoding gene to host tissue. 9
Gene Editing Tools
Conventional gene therapy mostly depends on viral-based delivery of genes that either randomly integrates into the host genome (eg retroviruses) or remains as extrachromosomal DNA copy (eg AAV]) and expresses a protein that is missing or mutated in human disorder. In contrast to traditional gene therapy, gene editing provides more versatile tools for gene therapy, for example, precisely correct point variants, place an extra, healthy gene at a safe genomic location or disrupt a gene. The Current gene-editing process depends on the introduction of endogenous double-strand DNA breaks (DSBs) and repair mechanisms. When DSBs occur by nucleases, cellular DNA repair mechanisms are activated. There are two main mechanisms for repairing double-strand breaks, non-homologous end joining (NHEJ) and homology-directed repair (HDR). Genome-editing nucleases can be modified to recognize and break the genome at specific DNA sequences, resulting in DSBs, which are efficiently repaired by either NHEJ or HDR. 10 , 11
NHEJ repair damaged DNA without a homologous template. Due to this reason, NHEJ may lead to deletions or insertions of nucleotides in the damaged loci; thus, it is error-prone. HDR differs from NHEJ since it repairs DNA damages using a homologous template. Generally, having used a homologous sequence, this form of DNA repair has less chance to cause errors. From a clinical viewpoint, HDR is favorable for restoring mutations in genes or for integrating genes for therapeutic purposes. 10–13
Currently, there are four different gene-editing nuclease enzymes available based on their structures: meganucleases, zinc-finger nucleases, transcription activator-like effector nucleases, and CRISPR-associated nucleases.
Meganucleases (MNs)
Are sequence-specific endonucleases that recognize unique large (14–40 bp) target sites. It has low cytotoxicity that makes it an attractive tool for genome editing. Existing engineering techniques include the creation of fusion protein from existing MN domains and engineering MN specificity via the direct alteration of protein residues in the DNA-binding domain. The complexity in re-engineering and low editing efficiency limits the uses of MNs. 14
Zinc Finger Nucleases (ZFNs)
Artificially produced by fusing site-specific zinc finger protein with the non-specific cleavage domain of the FokI restriction endonuclease. The DNA-binding component has 3–6 zinc finger repeats, and each can identify between 9 and 18 base pairs. ZFN has three zinc fingers that each identifies three base pair DNA sequence to form a three-finger array that attaches to nine base pair target sites and the non-specific cleavage domain. 14 , 15 ZFPs deliver a site-specific DSB to the genome and facilitate local homologous recombination that enhances targeted genome editing. The ZFN-encoding plasmid-based targeted administration of the required genes decreases the limitations of viral administration. If ZFNs are not specific at the target site, off-target break may occur. Such off-target breakage may cause DBS that causes cell death. An Off-target break may facilitate the random integration of donor DNA. 15 , 16
Transcription Activator-Like Effector Nucleases (TALENs)
Are artificial DNA nucleases formed by fusing a DNA-binding domain with a nonspecific nuclease domain derived from Fok I endonuclease that specifically cut the required DNA sequence. 15 TALE effectors DNA-binding domain has a repeating unit of 33–35 conserved amino acids. Each repeat is similar, except positions 12 and 13, which are variable and have a strong correlation with specific nucleotide recognition. DNA cleavage domain is nonspecific from FokI endonuclease. The FokI domain acts as a dimer that needs two constructs with unique DNA binding for sites in the target genome. Both the number of amino acids between the TALE DNA binding domain and the FokI cleavage domain are essential for better activity. TALEN uses to edit genomes by inducing DSB that cells respond to with repair mechanisms. 17 , 18
CRISPR is a heritable, adaptive immune system of bacteria that provides them with the memory of previous virus infections and defends against re-infection. Contrary to the human adaptive immune system, CRISPR is passed on to the next generation of bacteria, rendering the colony immune to future virus infections. CRISPR immunity depends on the integration of the invader’s DNA (virus or plasmid) into the bacterial genome. 19 CRISPR helps the bacterium to identify the viral sequences and break. CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats, which are interrupted by “spacer” sequences. These “spacer” sequences are viral sequences integrated during past viral infections when transcribed into short RNA sequences, are capable of guiding the Cas endonuclease to complementary sequences of viral DNA. Upon target identification, Cas binds to the viral DNA and cleaves it, protecting the prokaryotic cell from infection. 20 , 21 CRISPR immune system modified to create a gene-editing tool that can target changes to the DNA. The most common is CRISPR/Cas9, which posses the Cas9 endonuclease and a short noncoding guide RNA (gRNA) that contains two components: a target-specific CRISPR RNA (crRNA) and a helper trans-activating RNA (tracrRNA). The gRNA unit guides Cas9 to a specific genomic locus via base pairing between the crRNA sequence and the target sequence. 22 CRISPR-Cas-mediated gene repair, disruption, insertion, or deletion are thus finding applications in several areas of biomedical research, medicine, agriculture, and biotechnology. 22 , 23
Gene Delivery Technologies
Since the emergence of recombinant DNA technology that helps gene-therapy, how to effectively and safely administer gene products is the major challenge. Vector is a vehicle that uses to deliver the gene of interest. An ideal vector can administer a gene to a specific tissue, accommodate enough foreign gene size, achieve the level and duration of transgenic expression enough to correct the defect gene, non-immunogenic, and safe. Delivery of the gene products done by Viral Vectors, Bactofection, and none viral Vectors (chemical and physical) method as summarized in Figure 1 . 24 The most important step in achieving gene therapy is choosing the vectors.
Overview of the delivery systems used in gene therapy.
Viral Vectors Used for Gene Delivery
Viruses were the first and the most widely used vectors to administer genes into the target tissue. Viral vectors ensure that almost all cells can infect, without affecting cell viability. Viruses have distinctive features that make them suitable for gene delivery in clinical practice. Surface proteins on viruses interact with their host receptors, which activate endocytosis. Once entered, viruses release their genome into the nucleus for viral gene expression. 25 , 26 Herpes simplex virus (HSV), adenovirus (Ad), adeno-associated virus (AAV), and lentivirus (LV) are the most important viral vectors. 27 , 28
Bacterial Mediated Gene Transfer (Bactofection)
Some bacteria specifically target tumor cells leading to RNA interference (RNAi) and gene silencing by inhibiting RNA activity, such as protein synthesis. Several in vivo and in vitro studies revealed that intracellular bacteria such as Salmonella spp., Listeria monocytogenes, Shigella flexneri, Bifidobacterium longum, E. coli , and Yersinia enterocolitica use to deliver plasmids pro-drug converting enzymes and cytotoxic agents into the target cell. 29 Phase I trial is undergoing by using Listeria , Bifidobacterium , Salmonella , Shigella , and Clostridium gene therapy against cancer. Another clinical trial is ongoing on the effects of Lactococcus synthesizing interleukin 10 against colitis in Phase II. 30 , 31
Chemical-Based Nonviral Vectors
Viral-vectors-based gene transfer displays better and long-term gene encoding but has some limitations like immunogenicity, less specific to the target cell, carcinogenicity, high cost and cannot deliver large genome size. Non-viral methods display better advantages due to relatively safe, can deliver a large genome, and ease for production. 32–35 Chemical vectors, also known as non-viral vectors grouped as organic and inorganic vectors. The organic vectors consist of cationic lipid-based vectors: synthetic cationic polymers-based vector and peptide-based vectors. These cationic organic vectors form complexes with negatively charged DNA via an electrostatic bond. The complexes protect the genomic material and enhance cell uptake and intracellular delivery. Generally, non-viral vectors help to deliver small DNA, large DNA (plasmid DNA), and RNA (Si RNA, m RNA) into the target tissue. 36–38 Physical methods use different mechanical forces to facilitate the administration of gene material into the host tissues. It is an alternative to viral and chemical methods to decrease barriers that limit DNA delivery into the host tissues. 39 It is feasible to deliver genes into target tissues by mechanical force. Indeed, there are several methods, and most have a similar mode of gene delivery, ie, physically formed transient pores in the cell membrane through which the genetic material enters into the host cell. 40 , 41 Needle and jet injection, hydrodynamic gene transfer, electroporation, sonoporation, magnetofection, and gene gun bombardment are examples of physical DNA delivering methods. 42–44
Gene Therapy for Cancer Treatment
Cancer occurs due to disrupting the normal cell proliferation and apoptosis process. Advances in cancer therapy need a novel therapeutic agent with novel mode of action, several mechanisms of cell death, and synergy with conventional management. Gene therapies possess all these profiles. Several gene therapy approaches were developed for the management of cancer, including anti-angiogenic gene therapy, suicide gene therapy, immunotherapy, siRNA therapy, pro-apoptotic gene therapy, oncolytic virotherapy, and gene directed-enzyme prodrug therapy. 45 By November 2017, greater than 2597 clinical trials were conducted on gene therapy in the world. Among these trials, greater than 65% are associated with cancer, followed by monogenetic and cardiovascular diseases. 8 The use of CAR T cell therapy showed promising results for the management of both myeloid and lymphoid leukemia. Until August 2019, only 22 gene products were approved for the treatment of different disorders. Most gene products used for the treatment variety types of cancers as shown in Table 1 . Immuno-gene therapy is a potential treatment approach for the treatment of p53-deficient tumors (Imlygic, Gendicine, Yescarta, and Kymriah. 47
Gene Therapies Products Approved for Therapeutic Use
Oncolytic Virotherapy
Oncolytic virotherapy (OV) is the most promising approach for tumor immunotherapy. OV uses replication-competent viruses that can proliferate selectively at tumor cells. Oncolytic viruses grouped as naturally occurring or genetically modified viruses. Natural occurring viruses like parvoviruses, and Newcastle disease viruses that selectively replicate in tumor cell without genetic modification. The second virus category, such as vesicular stomatitis viruses, adenoviruses, measles viruses, HSV and vaccinia viruses, genetically modified to improve the safety, tumor-specificity, and decrease virus pathogenicity. The therapeutic use of oncolytic viruses for cancer treatment is an immune-related treatment alternative. Oncolytic viruses act by directly lyses tumor cells and by introducing wild-type tumor suppressor genes into cells that lack the tumor suppressor gene. 48 , 49 Change in p53 gene function is present in half of all malignancies, and the induction of wild-type p53 gene re-establishes the normal p53 expression. Several recombinant OVs expressing p53 were developed with the aim of producing more potent OVs that act in combination with host immunity or with other treatments’ modality to destroy tumor cells. 49 , 50
Gendicine (Recombinant Human P53 Adenovirus [Ad5RSV- P53 ])
Was the first approved gene product for the management of neck and head squamous cell carcinoma in 2003. 50 Gendicine is a non-replicative an adenoviral vector, where the E1 gene is replaced with the tumor suppressor p53 cDNA gene. The expression of p53 in tumor cells triggers the antitumor effect by activating the apoptotic pathway, inhibit damaged DNA repair, and anti-apoptotic activity. P53 gene mutation is prevalent in several cancers. Therefore, Gendicine induces the expression of p53 restores its activity and destroys the tumor cells. Generally, Gendicine management showed 30–40% complete response and 50–60% partial response with a total response rate of 90%–96% in different therapeutic use. Up-to-date greater than 30,000 patients managed by Gendicine. 50 , 51
Oncorine (rAd5-H101)
It is the first replicative, oncolytic recombinant ad5 (rAd5-H101) approved to treat refractory nasopharyngeal cancer. Loss of p53 gene linked with drug resistance and survival rate reduction in non-small cell cancer patients. 50 Oncorine is an ad5 virus with a deletion in the E1B 55K gene. Host cell p53 gene inactivation is essential for wild-type to block the activation of apoptotic pathway. The removal of the E1B 55K gene inhibits viral proliferation in normal cells, allowing only proliferate in p53-deficient host cells. In tumor cells, viral proliferation causes oncolysis that is the mechanism to treat solid tumors. Following cancer cell lysis, adenoviruses release and infect another cell activating a serious of Oncorine-mediated cell death. 52 , 53
Imlygic (Talimogene Laherparepvec)
It is a genetically modified oncolytic HSV-1 approved in Europe in 2015 for the management of non-resectable metastatic melanoma. Imlygic is the first oncolytic virus used for the management of advanced melanoma. 48 The replacement of γ34.5 and α47 genes with the human granulocyte-macrophage colony-stimulating factor (GM-CSF) gene modifies the HSV-1 gene. The γ34.5 gene deletion causes tumor cell-selective replication and suppression of pathogenicity. The γ34.5 gene blocks protein synthesis of the host cell during viral infection. Thus, suppressing γ34.5 seizes the virus proliferation in normal cells. In tumor cells, the γ34.5 gene deleted HSV-1 can replicate. The α47gene inhibits the host cell transporter associated with antigen presentation. The depletion of α47gene reduces MHC class I expression that increases antitumor immune activity. 53 Besides, two human GM-CSF genes inserted into the virus providing high levels of GM-CSF production, and stimulate immune responses. Administration of Imlygic causes apoptosis of tumor cell enhanced antigen presentation and increased antitumor response. 49 , 54
Rexin-G (Mx-dnG1)
Is the first targeted injectable vector approved for the management of metastatic cancers. It is a replication-incompetent retroviral vector showing a SIG -binding peptide to bind to abnormal Signature ( SIG ) proteins in the tumor cell that increase vector concentration in tumor cells and express a dominant-negative human cyclin G1 inhibitor. After the entrance into the tumor cells, Rexin-G synthesizes cytocidal dnG1 proteins that inhibit the cell cycle in the G1 phase resulting in apoptosis of cancer cells. 55 , 56
Chimeric Antigen Receptor (Car) T Cell Therapy
T cells destroy infected and tumor cells by detecting nonself antigens with the T cell receptor (TCR). CAR T is a T cell transduced with a chimeric antigen receptor specific to a tumor-associated antigen. CAR is “chimeric” because it contains the antigen-binding site of the B cell receptor and an intracellular TCR activation domain. CAR has three domains, an extracellular domain that has cancer-specific epitopes (scfv region) made from light (V L ) and heavy (V H ) chains of immunoglobin that target antigen (such as CD19), a transmembrane domain, and intracellular TCR derived stimulatory domains as showed in Figure 2 . The scfv component binds to the target antigen in the MHC independent way leading to CAR clustering and stimulating T-cell via intracellular region that posses the TCR-derived CD3ζ chain, with or without co-stimulatory domains. Stimulated CAR T-cells give target-specific memory cells that inhibit tumor relapse. 57 CD19‐targeted CAR T cells were the first CARs to be studied. CD19 is a promising target due to its expression limited to the B cell. First‐generation, CD19‐targeted CAR T cells were safe but ineffective. Second-generation CARs have a costimulatory domain with the CD3ζ activation domain show enhanced T cell activity. Two second‐generation, CD19‐targeted CARs are in clinical use contain a 41BB costimulatory domain (19‐BBz) and a CD28 costimulatory domain and those with more than one additional co-stimulatory molecule are known as “third-generation” CAR. 57–59
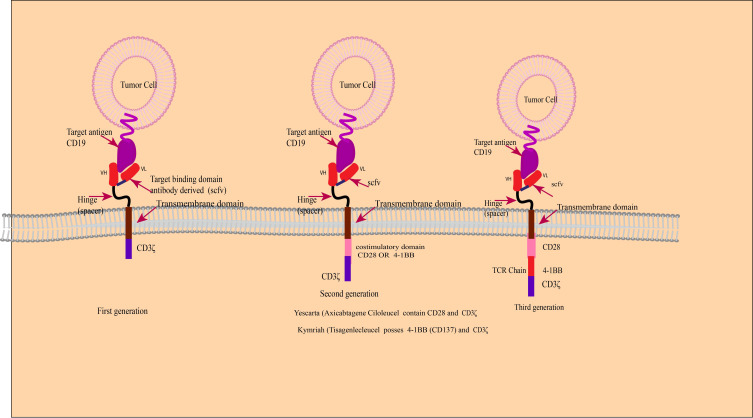
Schematic diagram of CAR-T-cell products.
Kymriah (Tisagenlecleucel)
It is the first FDA approved CAR T-cell-based gene product to treat relapsed B-cell acute lymphoblastic leukemia. Kymriah has autologous T cells, modified with the lent virus to encode a CAR consist of a murine single-chain antibody fragment (scFv) selective for CD19, an intracellular domain 4–1BB (CD137), and CD3 zeta with CD8 transmembrane hinge. After binding to CD19 antigen-expressing cells, Kymriah initiates the antitumor effect via the CD3 domain. The intracellular 4–1BB co-stimulatory domains enhance the antitumor activity. The CD19 antigen is a 95-kD glycoprotein encoded as a surface antigen in diffuse large B-cell lymphoma (DLBCL) and other B-cell lymphomas. 60 , 61 High response rates were recorded in patients with refractory DLBCL in Phase 2 clinical trials. The response rate was 50% at 3 months, 43% with a complete response at 6 months, and there were no patients with a complete response at 6 months who had a relapse by the median of 28.6 months. 62
Yescarta (Axicabtagene Ciloleucel)
It is another CAR T-cell therapy used for the management of aggressive non-Hodgkin lymphoma. It is CD19 antigen-specific ex-vivo modified autologous T cells infected with a gamma-retroviral. It encodes a CAR comprising an extracellular murine anti-CD19 single-chain variable fragment fused to a cytoplasmic domain that possesses CD28 and CD3-zeta co-stimulatory domains. 63 , 64
Zalmoxis (Allogenic T Cells Encoding LNGFR and HSV-TK)
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) uses for the management of several hematopoietic malignancies. But, acute graft-versus-host-disease (aGvHD) and Graft rejection are barriers to its success. The treatment strategy for haplo-HSCT depends on T-cell depletion or administration of lymphotoxin agents like cyclophosphamide after stem cell infusion to selectively deplete activated alloreactive lymphocytes but causes prolonged immunodeficiency post-transplantation. Thus, treatment to enhance immune reconstitution after transplantation is necessary. 65 Zalmoxis is a genetically modified allogeneic T cell using a retroviral vector encoding a human low-affinity nerve growth factor receptor (ΔLNGFR) and HSV-TK Mut2 to transduce the allogeneic T immune cells. The ΔLNGFR expression uses as a marker of the transduced T cells, and the HSV-TK Mut2 expression provides the suicide gene induction during the administration of the prodrug ganciclovir (GCV). Administration of the genetically modified donor T cells to T cell-depleted transplant patients (HSCT) reconstitutes the immunity to defend from infections. But, donor cells may specifically act as the host cells leading to Graft Versus Host Disease (GVHD). In this case, induction of suicide gene by GCV administration may kill the donor T cells encoding HSV-TK and control GVHD. Zalmoxis is a potential curative agent for HSCT patients when the matched donor does not exist. Zalmoxis provides post-transplant GvHD control, Graft versus Leukemia (GvL) improvement, relapse decrease, and immune reconstitution causes reduced infection. 52 , 66
Gene Silencing
Gene silencing therapy is RNA interference (RNAi)-mediated knockdown of specific genes in tumor cells. RNAi is single or double-stranded noncoding RNAs (21 ribonucleotides) that induce sequence-specific degradation of complementary mRNAs via the cells’ internal machinery. 67 siRNA is vital because most genes do not have inhibitors due to a lack of ligand binding sites and amino acid sequence homology with other proteins that limit target selectivity. RNAi consists of microRNA (miRNA), Small Interfering RNA (siRNA) and short hairpin RNA (shRNA). Two decades later after the discovery of RNAi, ONPATTRO™ (patisiran) approved for the first time for the management of the polyneuropathy of hereditary transthyretin‐mediated (hATTR) amyloidosis. 68 Tumor suppressor genes, oncogenes, genes involved in cancer progression, and drug-resistance are promising targets for gene silencing by RNAi-based cancer treatment due to selective gene silencing effect and relatively fewer adverse effects than conventional chemotherapy. 69 The merits of RNAi in cancer treatment are targeting several genes of different cellular pathways involved in cancer progression and develop a drug for a specific patient. 70 Several studies conducted on animals revealed that targeting vital proteins in the cell cycle, such as Protein kinase N3 (PKN3), kinesin spindle protein (KSP), and polo-like kinase 1 (PLK1) by siRNA displayed a potent antitumor effect. Several liposomal siRNA dose preparations are in Phase 1 trials, such as treatments for pancreatic cancer (PKN3 siRNA), liver cancer (CEBPA siRNA), and neuroendocrine tumors (PLK1 siRNA). 71
Suicide Gene Therapy
Suicide gene therapy uses viral or bacterial genes into malignant cells that metabolize non-toxic prodrug into a toxic compound. Several suicide gene systems were identified including the HSV-thymidine kinase gene (HSV-TK) with ganciclovir (GCV) and the cytosine deaminase gene (CD) with 5-fluorocytosine (5-FC). 72 Gene-mediated cytotoxic immunotherapy is one strategy where an adenoviral vector possessing the herpes virus thymidine kinase gene (AdV-TK) is administered locally into the tumor site that causes local expression of the HSV-TK gene to the synthesis of viral thymidine kinase that converts GCV to GCV monophosphate. The next step is the administration of GCV that is a substrate of HSV-TK and phosphorylated to produce GCV monophosphate. Then, cellular kinases metabolize GVC-monophosphate into GVC-triphosphate. GCV triphosphate is a deoxyguanosine triphosphate analog, incorporated into the DNA chain causing chain termination and tumor cell death. 73
The anti-tumor effect of the TK/GCV system showed promising results in animal models. A study on hormone-refractory prostate cancer patients treated with HSV-TK delivered by adenovirus followed by GCV. The result showed response was at the surrogate marker level and safe. Several studies are in Phase III trials. 74 The cytosine deaminase (CD) enzyme exists in fungi and bacteria but not in mammalian cells, metabolizes cytosine into uracil. CD metabolizes the non-toxic prodrug 5-FC into 5-FU, which is subsequently metabolized by cellular enzymes into 5-FdUMP, 5-FdUTP, and 5-FUTP. Inhibition of thymidylate synthase and production of (5-FU) DNA and RNA are the mode of cell death induced by the CD/5-FC suicide system. 5-FU uses for cancer treatment but requires a high dose. This suicide system results in tumor-targeted chemotherapy with few side effects. The CD/5-FC system improved by the inclusion uracil phosphoribosyltransferase (UPRT) gene that phosphorylates 5-FU to 5-fluorouridine mono-phosphate, the first step of its pathway to activation. 75 The anti-tumor effect of the CD/5-FC combination showed a better efficacy in animal models. A study on refractory cancer patients that involved intratumoral administration of TAPET-CD attenuated Salmonella bacterium encoding the E. coli CD gene in three patients. The study showed a significant effect and lack of side effects. An oncolytic adenovirus possessing a CD/HSV-1 TK gene was used in a phase I study in patients with prostate cancer. The result showed that the transgene encoding persistence in the prostate for 3 weeks after administration. 76
Anti-Tumor Angiogenesis
Tumor-driven angiogenesis several growth factors are involved, such as vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), angiopoietins or IL-8, to secure oxygen and nutrients supply. Two major approaches are being pursued to block tumor angiogenesis. The first approach is down-regulation of pro-angiogenic factors expression, such as VEGF, and the second approach is up-regulation of expression of anti-angiogenic factors such as angiostatin, endostatin, and human soluble FMS-like tyrosine kinase receptor. Despite the successful therapeutic use of mAb like Bevacizumab for targeted therapy of cancer, the production and administration of therapeutic mAb are limited due to costly production. Therefore, gene-based studies were done to develop an angiogenesis-targeted cancer treatment. 77 , 78
Gene therapy represents a novel alternative for the management of diseases that have no satisfactory cure. Gene therapy for cancer treatment has good progress in the last three decades, few drugs approved, while others are still in trials. Relatively gene therapy has better safety with tolerable adverse effects than chemotherapy for the treatment of cancer. In the future, tumor genomic analysis, assessment of host humoral and cellular immunity will facilitate a better selection of the most appropriate patient for gene therapy. Recent progress in developing safe and effective vectors for gene delivery, and understanding the activity of nucleases facilitate future genome editing as new treatment approaches for untreatable diseases like cancer.
The success of using autologous and allogenic chimeric antigen receptor integrated T-lymphocytes in mediating adoptive immunotherapy enhances the safety and effectiveness of gene therapy. Besides, the enhanced biological research, cheaper gene vectors will be available in the market, which increases gene therapy accessibility for most cancer patients. This will change the future of cancer treatment, from generalized cancer treatment strategies to individualized cancer treatment, based on the patient’s specific genome, immune status, and genetic profile of the tumor. Gene therapy is expected to be fast, effective, less toxic, and inexpensive, with higher cure rates. In November 2017, more than 2597 clinical trials are ongoing in several countries and a few of them are listed in Table 2 . Until August 2019, 22 gene medicines had been approved by the drug regulatory agencies from various countries. 79 Gene therapy gradually accepted by the government and the public since the 1980s and has become an important alternative to the existing treatments in the past few years. Therefore, gene therapy drugs, with safe vectors and advanced biotechnologies, would play a greater role in the prophylaxis and management of cancer in the future.
Gene Therapies Products Candidates Under Clinical Trial
Acknowledgment
I would like to acknowledge Mrs Fasika Abu for editing the manuscript for English Style.
Abbreviations
ADA, adenosine deaminase; Ad, adenovirus; AAV, adeno-associated virus; aGvHD, acute graft-versus-host-disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; CAR, chimeric antigen receptor; DSBs, double-strand breaks; ERT, enzyme replacement therapy; HDR, homology-directed repair; HSV, herpes simplex virus; IRDs, inherited retinal degenerations; LV, lentivirus; NHEJ, non-homologous end joining; NMDs, neuromuscular disorders; OV, oncolytic virotherapy; tracrRNA, trans-activating RNA; TCR, T cell receptor; MNs, meganucleases.
Data Sharing Statement
All data are provided in the manuscript or found from published papers as cited.
The authors declare no competing interests in this work.
- 1. Wirth T, Parker N, Ylä-Herttuala S. History of gene therapy. Gene . 2013;525(2):162–169. doi: 10.1016/j.gene.2013.03.137 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Sharma AR, Kundu SK, Nam JS, et al. Next generation delivery system for proteins and genes of therapeutic purpose: why and how? Biomed Res Int . 2014;2014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Templeton NS, editor. Gene and Cell Therapy: Therapeutic Mechanisms and Strategies . Crc Press; 2008. [ Google Scholar ]
- 4. Vermezovic J, Stergiou L, Hengartner MO, Di Fagagna FD. Differential regulation of DNA damage response activation between somatic and germline cells in Caenorhabditis elegans. Cell Death Differ . 2012;19(11):1847–1855. doi: 10.1038/cdd.2012.69 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Reis RL. Encyclopedia of Tissue Engineering and Regenerative Medicine . Academic Press; 2019. [ Google Scholar ]
- 6. Colella P, Ronzitti G, Mingozzi F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol Ther Methods Clin Dev . 2018;8:87–104. doi: 10.1016/j.omtm.2017.11.007 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Merten O-W, Charrier S, Laroudie N, et al. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum Gene Ther . 2011;22(3):343–356. doi: 10.1089/hum.2010.060 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med . 2018;20(5):e3015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Sung YK, Kim SW. The practical application of gene vectors in cancer therapy. Integrat Cancer Sci Therap . 2018;5:1–5. [ Google Scholar ]
- 10. Suleiman AA, Saedi WY, Muhaidi MJ. Widely used gene editing strategies in cancer treatment a systematic review. Gene Rep . 2020;100983. [ Google Scholar ]
- 11. Zhang HX, Zhang Y, Yin H. Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol Therapy . 2019;27(4):735–746. doi: 10.1016/j.ymthe.2019.01.014 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Ernst MP, Broeders M, Herrero-Hernandez P, Oussoren E, van der Ploeg AT, Pijnappel WP. Ready for repair? Gene editing enters the clinic for the treatment of human disease. Mol Therapy Methods Clin Dev . 2020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Sallmyr A, Tomkinson AE. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J Biol Chem . 2018;293(27):10536–10546. doi: 10.1074/jbc.TM117.000375 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Zaman QU, Li C, Cheng H, Hu Q. Genome editing opens a new era of genetic improvement in polyploid crops. Crop J . 2019;7(2):141–150. doi: 10.1016/j.cj.2018.07.004 [ DOI ] [ Google Scholar ]
- 15. Palpant NJ, Dudzinski D. Zinc finger nucleases: looking toward translation. Gene Ther . 2013;20(2):121–127. doi: 10.1038/gt.2012.2 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Cassandri M, Smirnov A, Novelli F, et al. Zinc-finger proteins in health and disease. Cell Death Discovery . 2017;3(1):1–2. doi: 10.1038/cddiscovery.2017.71 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Sun N, Zhao H. Transcription activator‐like effector nucleases (TALENs): a highly efficient and versatile tool for genome editing. Biotechnol Bioeng . 2013;110(7):1811–1821. doi: 10.1002/bit.24890 [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Nakano C, Kitabatake Y, Takeyari S, et al. Genetic correction of induced pluripotent stem cells mediated by transcription activator-like effector nucleases targeting ALPL recovers enzyme activity and calcification in vitro. Mol Genet Metab . 2019;127(2):158–165. doi: 10.1016/j.ymgme.2019.05.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Hille F, Richter H, Wong SP, Bratovič M, Ressel S, Charpentier E. The biology of CRISPR-Cas: backward and forward. Cell . 2018;172(6):1239–1259. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Rath D, Amlinger L, Rath A, Lundgren M. The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie . 2015;117:119–128. doi: 10.1016/j.biochi.2015.03.025 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Terns RM, Terns MP. CRISPR-based technologies: prokaryotic defense weapons repurposed. Trends Genetics . 2014;30(3):111–118. doi: 10.1016/j.tig.2014.01.003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Li Y, Glass Z, Huang M, Chen ZY, Xu Q. Ex vivo cell-based CRISPR/Cas9 genome editing for therapeutic applications. Biomaterials . 2020;234:119711. doi: 10.1016/j.biomaterials.2019.119711 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell . 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Mali S. Delivery systems for gene therapy. Indian J Hum Genet . 2013;19(1):3. doi: 10.4103/0971-6866.112870 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomaterials Res . 2019;23(1):8. doi: 10.1186/s40824-019-0156-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Humphreys IR, Sebastian S. Novel viral vectors in infectious diseases. Immunology . 2018;153(1):1–9. doi: 10.1111/imm.12829 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Bouard D, Alazard‐Dany N, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol . 2009;157(2):153–165. doi: 10.1038/bjp.2008.349 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Goswami R, Subramanian G, Silayeva L, et al. Gene therapy leaves a vicious cycle. Front Oncol . 2019;9:297. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Pilgrim S, Stritzker J, Schoen C, et al. Bactofection of mammalian cells by Listeria monocytogenes: improvement and mechanism of DNA delivery. Gene Ther . 2003;10(24):2036–2045. doi: 10.1038/sj.gt.3302105 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Johnson SA, Ormsby MJ, McIntosh A, Tait SW, Blyth K, Wall DM. Increasing the bactofection capacity of a mammalian expression vector by removal of the f1 ori. Cancer Gene Ther . 2019;26(7):183–194. doi: 10.1038/s41417-018-0039-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Celec P, Gardlik R. Gene therapy using bacterial vectors. Front Biosci . 2017;22:81–95. doi: 10.2741/4473 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Wang W, Li W, Ma N, Steinhoff G. Non-viral gene delivery methods. Curr Pharm Biotechnol . 2013;14(1):46–60. [ PubMed ] [ Google Scholar ]
- 33. Ramamoorth M, Narvekar A. Non viral vectors in gene therapy-an overview. J Clin Diagnostic Res . 2015;9(1):GE01. doi: 10.7860/JCDR/2015/10443.5394 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Buck J, Grossen P, Cullis PR, Huwyler J, Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano . 2019;13(4):3754–3782. doi: 10.1021/acsnano.8b07858 [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Yang R, Chen F, Guo J, Zhou D, Luan S. Recent advances in polymeric biomaterials-based gene delivery for cartilage repair. Bioactive Mater . 2020;5(4):990–1003. doi: 10.1016/j.bioactmat.2020.06.004 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Van Bruggen C, Hexum JK, Tan Z, Dalal RJ, Reineke TM. Nonviral gene delivery with cationic glycopolymers. Acc Chem Res . 2019;52(5):1347–1358. doi: 10.1021/acs.accounts.8b00665 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Darr JA, Zhang J, Makwana NM, Weng X. Continuous hydrothermal synthesis of inorganic nanoparticles: applications and future directions. Chem Rev . 2017;117(17):11125–11238. doi: 10.1021/acs.chemrev.6b00417 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Riley MK, Vermerris W. Recent advances in nanomaterials for gene delivery—a review. Nanomaterials . 2017;7(5):94. doi: 10.3390/nano7050094 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Alsaggar M, Liu D. Physical Methods for Gene Transfer. In Advances in Genetics . Vol. 89. Academic Press; 2015:1–24. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Li X, Ruddy B, Taberner A. Characterization of needle-assisted jet injections. J Controlled Release . 2016;243:195–203. doi: 10.1016/j.jconrel.2016.10.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Therapy . 2007;15(12):2063–2069. doi: 10.1038/sj.mt.6300314 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Herrero MJ, Sendra L, Miguel A, Aliño SF. Physical Methods of Gene Delivery. In Safety and Efficacy of Gene-Based Therapeutics for Inherited Disorders . Cham: Springer; 2017:113–135. [ Google Scholar ]
- 43. Du X, Wang J, Zhou Q, et al. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv . 2018;25(1):1516–1525. doi: 10.1080/10717544.2018.1480674 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Smolders S, Kessels S, Smolders SM, et al. Magnetofection is superior to other chemical transfection methods in a microglial cell line. J Neurosci Methods . 2018;293:169–173. doi: 10.1016/j.jneumeth.2017.09.017 [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett . 2018;16(1):687–702. doi: 10.3892/ol.2018.8733 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther . 2021;29(2):464–488. doi: 10.1016/j.ymthe.2020.12.007 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Tristán-Manzano M, Justicia-Lirio P, Maldonado-Pérez N, Cortijo-Gutiérrez M, Benabdellah K, Martin F. Externally-controlled systems for immunotherapy: from bench to bedside. Front Immunol . 2020;11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Bommareddy PK, Patel A, Hossain S, Kaufman HL. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am J Clin Dermatol . 2017;18(1):1–5. doi: 10.1007/s40257-016-0238-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Sostoa JD, Dutoit V, Migliorini D. Oncolytic viruses as a platform for the treatment of malignant brain tumors. Int J Mol Sci . 2020;21(20):7449. doi: 10.3390/ijms21207449 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Zhang -W-W, Li L, Li D, et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum Gene Ther . 2018;29(2):160–179. doi: 10.1089/hum.2017.218 [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Xia Y, Li X, Sun W. Applications of recombinant adenovirus-p53 gene therapy for cancers in the clinic in China. Curr Gene Ther . 2020;20(2):127–141. doi: 10.2174/1566523220999200731003206 [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Shahryari A, Saghaeian Jazi M, Mohammadi S, Razavi Nikoo H, Nazari Z, Hosseini ES. Development and clinical translation of approved gene therapy products for genetic disorders. Front Genet . 2019;10:868. doi: 10.3389/fgene.2019.00868 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Russell L, Peng K-W. The emerging role of oncolytic virus therapy against cancer. Chine Clin Oncol . 2018;7(2):16. doi: 10.21037/cco.2018.04.04 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Osman AE, Luke JJ. The impact of the fecal microbiome on cancer immunotherapy. BioDrugs . 2019;33(1)::1–7. doi: 10.1007/s40259-018-0328-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Chawla SP, Bruckner H, Morse MA, Assudani N, Hall FL, Gordon EM. A Phase I-II study using rexin-g tumor-targeted retrovector encoding a dominant-negative cyclin g1 inhibitor for advanced pancreatic cancer. Mol Therapy Oncolytics . 2019;12:56–67. doi: 10.1016/j.omto.2018.12.005 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer. Expert Opin Biol Ther . 2010;10(5):819–832. doi: 10.1517/14712598.2010.481666 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Bb Pettitt D, Arshad Z, Smith J, Stanic T, Holländer G, Brindley D. CAR-T cells: a systematic review and mixed methods analysis of the clinical trial landscape. Mol Therapy . 2018;26(2):342–353. doi: 10.1016/j.ymthe.2017.10.019 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Oluwole OO, Davila ML. At The Bedside: clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J Leukoc Biol . 2016;100(6):1265–1272. doi: 10.1189/jlb.5BT1115-524R [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Ali S, Kjeken R, Niederlaender C, et al. The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the Treatment of Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma. oncologist . 2020;25(2):e321. doi: 10.1634/theoncologist.2019-0233 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev . 2016;30(3):157–167. doi: 10.1016/j.blre.2015.10.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Liu Y, Chen X, Han W, Zhang Y. Tisagenlecleucel, an approved anti-CD19 chimeric antigen receptor T-cell therapy for the treatment of leukemia. Drugs Today . 2017;53(11):597. doi: 10.1358/dot.2017.53.11.2725754 [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Eng J Med . 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Bouchkouj N, Kasamon YL, de Claro RA, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin Cancer Res . 2019;25(6):1702–1708. doi: 10.1158/1078-0432.CCR-18-2743 [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Jacobson CA, Farooq U, Ghobadi A. Axicabtagene ciloleucel, an Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for Relapsed or Refractory Large B-cell lymphoma: practical implications for the community oncologist. Oncologist . 2020;25(1):e138. doi: 10.1634/theoncologist.2019-0395 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Mohty M, Labopin M, Velardi A, et al. Allogeneic genetically modified T Cells (HSV-TK) as adjunctive treatment in haploidentical hematopoietic stem-cell transplantation (haplo-HSCT) of adult patients with high-risk hematological malignancies: a pair-matched analysis from the acute leukemia working party of EBMT. 2016. 672.
- 66. Hoogendoorn KH. Advanced Therapies: clinical, Non-clinical and Quality. Pharm Biotechn . 2019;357. [ Google Scholar ]
- 67. Parashar D, Rajendran V, Shukla R, Sistla R. Lipid-based nanocarriers for delivery of small interfering RNA for therapeutic use. Eur J Pharm Sci . 2020;142:105159. doi: 10.1016/j.ejps.2019.105159 [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Takeshita F, Ochiya T. Therapeutic potential of RNA interference against cancer. Cancer Sci . 2006;97(8):689–696. doi: 10.1111/j.1349-7006.2006.00234.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Hu B, Weng Y, Xia X-H, Liang X-J, Huang Y. Clinical advances of siRNA therapeutics. J Gene Med . 2019;21(7):e3097. doi: 10.1002/jgm.3097 [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Signal Transduction Targeted Ther . 2020;5(1):1–25. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Hossen MN, Wang L, Chinthalapally HR, et al. Switching the intracellular pathway and enhancing the therapeutic efficacy of small interfering RNA by auroliposome. Sci Adv . 2017;22(1):eaba5379. doi: 10.1126/sciadv.aba5379 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Düzgüneş N. Origins of Suicide Gene Therapy. In Suicide Gene Therapy . New York, NY: Humana Press; 2019:1–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Dey D, Evans GR. Suicide gene therapy by herpes simplex virus-1 thymidine kinase (HSV-TK). Targets Gene Therapy . 2011;65. [ Google Scholar ]
- 74. Sonabend AM, Ulasov IV, Lesniak MS. Gene therapy trials for the treatment of high-grade gliomas. Gene Ther Mol Biol . 2007;11(A):79. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Aučynaitė A, Rutkienė R, Tauraitė D, Meškys R, Urbonavičius J. Discovery of bacterial deaminases that convert 5-fluoroisocytosine into 5-fluorouracil. Front Microbiol . 2018;9:2375. doi: 10.3389/fmicb.2018.02375 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Zarogoulidis P, Darwiche K, Sakkas A, et al. Suicide gene therapy for cancer–current strategies. J Genet Syndr Gene Ther . 2013;4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. El‐Kenawi AE, El‐Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol . 2013;170(4):712–729. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett . 2018;16(1):687–702. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv . 2020;40:107502. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.8 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO