- Users' Guide to the Medical Literature
Explore the foundations of evidence-based medicine with JAMA’s Users’ Guide to the Medical Literature collection. Learn to understand and interpret clinical research!

Publication
Article type.
This Users’ Guide to the Medical Literature describes the fundamental concepts of platform trials and master protocols and reviews issues in the conduct and interpretation of these studies.
This Users’ Guide to the Medical Literature provides suggestions for understanding guideline methods and recommendations for clinicians seeking direction in evaluating clinical practice guidelines for potential use in their practice.
- Evaluating Machine Learning Articles JAMA Opinion November 12, 2019 Artificial Intelligence Full Text | pdf link PDF
This Users’ Guide to the Medical Literature discusses the use of machine learning models as a diagnostic tool, and it explains the important steps needed for making these models and the outcomes they derive clinically effective.
This Users’ Guide to the Medical Literature discusses discrimination and calibration, 2 primary ways to measure and compare the accuracy of clinical risk prediction models.
This Users’ Guide to the Medical Literature discusses strategies for adjusting analyses as a way of addressing prognostic imbalance in studies of therapy and harm.
- How to Read a Systematic Review and Meta-analysis and Apply the Results to Patient Care: Users’ Guides to the Medical Literature JAMA Review July 9, 2014 Surgery Ischemic Heart Disease Perioperative Care and Consultation Acute Coronary Syndromes Cardiology Full Text | pdf link PDF has multimedia
Sun and coauthors provide 5 criteria to help clinicians distinguish credible subgroup analyses from spurious subgroup analyses.
- How to Use an Article About Quality Improvement JAMA Review November 24, 2010 Health Care Quality Full Text | pdf link PDF
- How to Use an Article About Genetic Association: C: What Are the Results and Will They Help Me in Caring for My Patients? JAMA Review January 21, 2009 Genetics and Genomics Full Text | pdf link PDF
- How to Use an Article About Genetic Association: B: Are the Results of the Study Valid? JAMA Review January 14, 2009 Genetics and Genomics Full Text | pdf link PDF
- How to Use an Article About Genetic Association: A: Background Concepts JAMA Review January 7, 2009 Genetics and Genomics Full Text | pdf link PDF
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Published: 16 January 2023
The next generation of evidence-based medicine
- Vivek Subbiah ORCID: orcid.org/0000-0002-6064-6837 1 , 2 , 3
Nature Medicine volume 29 , pages 49–58 ( 2023 ) Cite this article
161k Accesses
181 Citations
785 Altmetric
Metrics details
- Adaptive clinical trial
- Drug development
- Health policy
Recently, advances in wearable technologies, data science and machine learning have begun to transform evidence-based medicine, offering a tantalizing glimpse into a future of next-generation ‘deep’ medicine. Despite stunning advances in basic science and technology, clinical translations in major areas of medicine are lagging. While the COVID-19 pandemic exposed inherent systemic limitations of the clinical trial landscape, it also spurred some positive changes, including new trial designs and a shift toward a more patient-centric and intuitive evidence-generation system. In this Perspective, I share my heuristic vision of the future of clinical trials and evidence-based medicine.
Similar content being viewed by others

Developing robust benchmarks for driving forward AI innovation in healthcare
Causal inference and counterfactual prediction in machine learning for actionable healthcare
Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management
The last 30 years have witnessed breathtaking, unparalleled advancements in scientific research—from a better understanding of the pathophysiology of basic disease processes and unraveling the cellular machinery at atomic resolution to developing therapies that alter the course and outcome of diseases in all areas of medicine. Moreover, exponential gains in genomics, immunology, proteomics, metabolomics, gut microbiomes, epigenetics and virology in parallel with big data science, computational biology and artificial intelligence (AI) have propelled these advances. In addition, the dawn of CRISPR–Cas9 technologies has opened a tantalizing array of opportunities in personalized medicine.
Despite these advances, their rapid translation from bench to bedside is lagging in most areas of medicine and clinical research remains outpaced. The drug development and clinical trial landscape continues to be expensive for all stakeholders, with a very high failure rate. In particular, the attrition rate for early-stage developmental therapeutics is quite high, as more than two-thirds of compounds succumb in the ‘valley of death’ between bench and bedside 1 , 2 . To bring a drug successfully through all phases of drug development into the clinic costs more than 1.5–2.5 billion dollars (refs. 3 , 4 ). This, combined with the inherent inefficiencies and deficiencies that plague the healthcare system, is leading to a crisis in clinical research. Therefore, innovative strategies are needed to engage patients and generate the necessary evidence to propel new advances into the clinic, so that they may improve public health. To achieve this, traditional clinical research models should make way for avant-garde ideas and trial designs.
Before the COVID-19 pandemic, the conduct of clinical research had remained almost unchanged for 30 years and some of the trial conduct norms and rules, although archaic, were unquestioned. The pandemic exposed many of the inherent systemic limitations in the conduct of trials 5 and forced the clinical trial research enterprise to reevaluate all processes—it has therefore disrupted, catalyzed and accelerated innovation in this domain 6 , 7 . The lessons learned should help researchers to design and implement next-generation ‘patient-centric’ clinical trials.
Chronic diseases continue to impact millions of lives and cause major financial strain to society 8 , but research is hampered by the fact that most of the data reside in data silos. The subspecialization of the clinical profession has led to silos within and among specialties; every major disease area seems to work completely independently. However, the best clinical care is provided in a multidisciplinary manner with all relevant information available and accessible. Better clinical research should harness the knowledge gained from each of the specialties to achieve a collaborative model enabling multidisciplinary, high-quality care and continued innovation in medicine. Because many disciplines in medicine view the same diseases differently—for example, infectious disease specialists view COVID-19 as a viral disease while cardiology experts view it as an inflammatory one—cross-discipline approaches will need to respect the approaches of other disciplines. Although a single model may not be appropriate for all diseases, cross-disciplinary collaboration will make the system more efficient to generate the best evidence.
Over the next decade, the application of machine learning, deep neural networks and multimodal biomedical AI is poised to reinvigorate clinical research from all angles, including drug discovery, image interpretation, streamlining electronic health records, improving workflow and, over time, advancing public health (Fig. 1 ). In addition, innovations in wearables, sensor technology and Internet of Medical Things (IoMT) architectures offer many opportunities (and challenges) to acquire data 9 . In this Perspective, I share my heuristic vision of the future of clinical trials and evidence generation and deliberate on the main areas that need improvement in the domains of clinical trial design, clinical trial conduct and evidence generation.
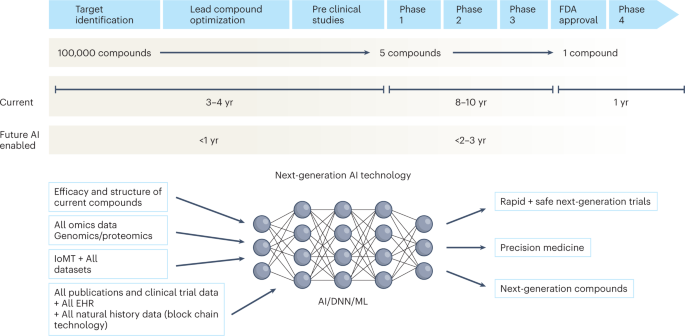
The figure represents the timeline from drug discovery to first-in-human phase 1 trials and ultimately FDA approval. Phase 4 studies occur after FDA approval and can go on for several years. There is an urgent need to reinvigorate clinical trials through drug discovery, interpreting imaging, streamlining electronic health records, and improving workflow, over time advancing public health. AI can aid in many of these aspects in all stages of drug development. DNN, deep neural network; EHR, electronic health records; IoMT, internet of medical things; ML, machine learning.
Clinical trial design
Trial design is one of the most important steps in clinical research—better protocol designs lead to better clinical trial conduct and faster ‘go/no-go’ decisions. Moreover, losses from poorly designed, failed trials are not only financial but also societal.
Challenges with randomized controlled trials
Randomized controlled trials (RCTs) have been the gold standard for evidence generation across all areas of medicine, as they allow unbiased estimates of treatment effect without confounders. Ideally, every medical treatment or intervention should be tested via a well-powered and well-controlled RCT. However, conducting RCTs is not always feasible owing to challenges in generating evidence in a timely manner, cost, design on narrow populations precluding generalizability, ethical barriers and the time taken to conduct these trials. By the time they are completed and published, RCTs become quickly outdated and, in some cases, irrelevant to the current context. In the field of cardiology alone, 30,000 RCTs have not been completed owing to recruitment challenges 10 . Moreover, trials are being designed in isolation and within silos, with many clinical questions remaining unanswered. Thus, traditional trial design paradigms must adapt to contemporary rapid advances in genomics, immunology and precision medicine 11 .
Progress in clinical trial design
High-quality evidence is needed for clinical practice, which has traditionally been achieved with RCTs 12 . In the last decade, substantial progress has been made in the design, conduct and implementation of ‘master’ protocols (overarching protocols that apply to several substudies), which has led to many practice changes that have substantially improved the stagnation of RCTs. Moreover, master protocols may involve parallel interventional studies in a single disease or multiple diseases defined by a biomarker or disease entity 12 . Four different classes of studies are included under the master protocols—the umbrella study, basket study, platform study and master observational trial (MOT) (Fig. 2 ). Each of these is a unique trial design that can include independent arms with control interventions and may be analyzed individually and/or collectively, with added flexibility 13 , 14 . The field of oncology has led these efforts more so than any other field, owing to advances in genomics (for identifying molecular alterations), discovery of therapeutics and rapid clinical translation, thus ushering in the precision oncology era.
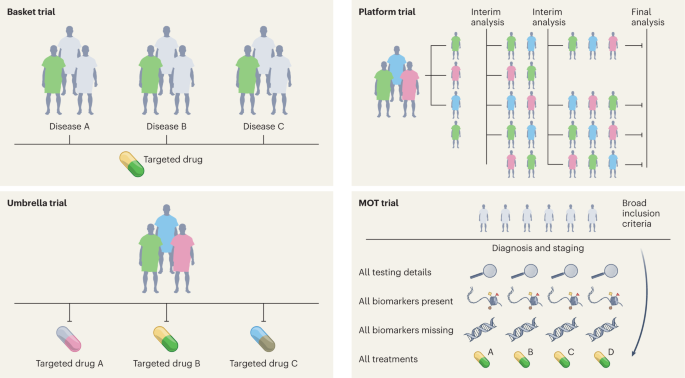
Four different classes of studies are included under the master protocols—the basket study, umbrella study, platform study and MOT.
Umbrella study
Umbrella trials are study designs that evaluate multiple targeted therapies for the same disease entity, stratified by molecular alteration. Examples include the I-SPY (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And Molecular Analysis) breast cancer trial and Lung-MAP (Lung Cancer Master Protocol) 15 , 16 .
Basket (or bucket) trial
Basket trials are tissue-agnostic or histology-independent studies where targeted therapy is evaluated on multiple disease types that all harbor the same underlying molecular aberration. For instance, the VE-Basket study (in which VE denotes vemurafenib) 17 , Rare Oncology Agnostic Research (ROAR) study 18 , ARROW trial 19 and LIBRETTO-001 trials 20 , 21 have led to several drug approvals in specific biomarker-driven populations in a histology-dependent and histology-independent manner.
Platform study
These are multi-arm, multistage study designs that compare several intervention groups with a common control group in the context of the same master protocol. Additionally, they can be perpetual/immortal study designs (with no defined end date) and are more efficient than traditional trials on account of the shared control arm, which ensures that a greater proportion of patients are enrolled in the interventional/experimental arms than in the control arm. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) Platform Study is a prominent example; this practice-changing trial established dexamethasone as an effective treatment for COVID-19 (ref. 22 ) and also showed that hydroxychloroquine was ineffective. Platform studies are flexible by design and do not necessarily need to have a shared control arm; the main idea is that intervention arms may be added to an ongoing trial, for example, as in the The UK Plasma Based Molecular Profiling of Advanced Breast Cancer to Inform Therapeutic CHoices (plasmaMATCH) platform trial 23 . Although the aforementioned trials were designed in the context of drug development in oncology and infectious diseases, the scope of platform trials could be leveraged in other diverse areas such as clinical psychology and neurology 24 . Such trials could also be used for digital mental health interventions and could be readily implemented in resource-constrained settings 24 .
The MOT is a prospective, observational study design that broadly accepts patients independently of biomarker signature and collects comprehensive data on each participant 14 , 25 . The MOT is a combination of the master interventional trial and prospective observational trial designs and attempts to hybridize the power of biomarker-based master interventional protocols with the breadth of real-world data (RWD) 14 , 25 . This approach could be well suited to collect prospective RWD across many specialties; the Registry of Oncology Outcomes Associated with Testing and Treatment (ROOT) MOT is one example 14 .
Development of biomarkers and defining endpoints
Biomarker development has facilitated progress in clinical trial design, with unprecedented advances in genomics and immunology leading to several approvals for biomarker-based targeted therapies and immunotherapy in the last decade. In fact, human genetics evidence provided support for more than two-thirds of the drug approvals in 2021 (ref. 26 ). The fields of oncology and genetics have benefited immensely from these advances, but fields such as cardiology, nephrology and pulmonology are still lagging in biomarker-based drug approvals.
To fast-track drug development and clinical trials in every major disease, we will need to define biomarkers (whether clinical, pathological or physiological) and their context of use for every disease process and delineate clear endpoints for studies 27 . Biomarkers can be diagnostic, prognostic or predictive and can inform early drug development, dose selection and trial design. In addition, biomarkers can help to fast-track basic science and drug discovery—all with the eventual goal of improving patient health 28 . However, the level of evidence for a biomarker largely depends on the context of use.
In addition to biomarkers, every field needs to define areas of top priority for research and identify the most relevant endpoints to answer priority research questions. Endpoints are measures of health and/or disease and serve different purposes depending on the phase of the trial 28 , 29 . Beyond clinical and regulatory endpoints, patient-reported outcomes and digital endpoints are also rapidly emerging.
Digital endpoints
Digital endpoints are sensor-generated data collected outside the clinical environment in the context of patients’ routine living—such as using smartphone microphones to monitor cognitive decline in people with Alzheimer’s disease or smartwatch monitors to evaluate drug effect in people with sickle-cell anemia 29 . This is an area of considerable excitement in medicine as it could permit more realistic real-world tracking of the patient experience. Moreover, with the increase in decentralized trial conduct across many specialties, remote monitoring is poised to increase. For instance, a recent study developed an AI model to detect and track progression of Parkinson’s disease (for which there are no biomarkers) on the basis of nocturnal breathing signals using noninvasive, at-home assessment, providing evidence that AI may be useful in risk assessment before clinical diagnosis of the condition 29 , 30 . Additionally, digital atrial fibrillation screening by smart devices has been evaluated extensively in large-scale studies, including the Apple 31 , Huawei 32 and Fitbit 33 cardiac studies. Altogether, these siteless observational studies enrolled over 1 million participants, an amazing feat, and a randomized study showed the superiority of digital atrial fibrillation detection over usual care 34 .
Digital characterization and assessment of clinical status need to be standardized and harmonized, with interdisciplinary collaboration and regulatory input. Consensus is also needed to identify and characterize intermediate and surrogate endpoints for major chronic diseases. This requires specialty-specific incorporation of multiple levels of data such as genomic, proteomic and genotype–phenotype-based clinical data and disease-specific measurements, in addition to a layer of functional data 26 . The National Institutes of Health (NIH) and Food and Drug Administration (FDA) have developed BEST (Biomarkers, EndpointS and other Tools) resources to clarify the ambiguity in biomarkers and endpoints. This is a ‘living document’ that is continually updated as standards and evidence change 35 and that clarifies important definitions and describes some of the hierarchical relationships, connections and dependencies among the terms.
Clinical trial conduct
The components of clinical trial conduct are protocol implementation; patient selection, recruitment, monitoring and retention; ensuring compliance to safety reporting; and continuing review and data analysis. The pharmaceutical industry and the healthcare sector invest substantial resources into clinical trial conduct, but changes are urgently needed to make the process more seamless. Moreover, the pace at which clinical trials are conducted is too slow to match the research advances in every field; thus, a high-tech transformation of every component in a stepwise manner is needed.
One of the positive sides of the pandemic is that it forced the system to redirect clinical trials to be more patient-centric than before, thus giving more importance to the principal subject of clinical research—the patient 36 (Fig. 3 ). This has led to decentralized trials and digital, remote and ‘virtual’ trials (which allow patients access to trials regardless of their geographic location), as well as ‘hospital-at-home’ and home-based monitoring concepts 37 . Such rapid strides have been aided by guidance from regulatory authorities 38 . Adopting an AI-based approach to enhance the patient experience can further improve high-fidelity assessments and ensure compliance with protocols 39 . Although digitalization, virtualization and decentralization are not cures for clinical research crises, they can create efficiencies that may have a sizeable and long-term downstream impact.
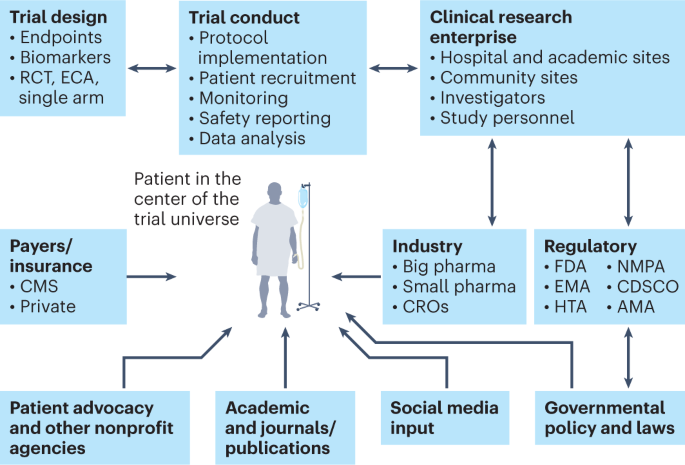
The main constituents of the clinical trial enterprise—patients, academic centers, industry sponsors (big and small pharma), government/cooperative group sponsors, regulatory agencies, patient advocacy organizations and CROs—need to work together, with the patient as the center of this clinical trial universe. AMA, African Medicines Agency; CDSCO, Central Drugs Standard Control Organization (India); CMS, Centers for Medicare and Medicaid Services; ECA, external control arm; EMA, European Medicines Agency; HTA, Health Technology Assessment; NMPA, National Medical Products Administration (China).
Physicians, healthcare team members and clinical investigators at academic sites and other trial enrolling sites contribute immensely to patient recruitment. In addition, high-impact, high-functioning sites (as in major academic centers of excellence) often have a portfolio of trials and screen patients presenting to the system in an efficient manner. Such sites are in the minority, however, and most clinical trial sites are challenged with staffing constraints and other barriers.
Clinical trial research enterprise
Efficiency and collaboration in the clinical trial research enterprise are major components of clinical trial success. The main constituents of the clinical trial enterprise are patients, academic centers, industry sponsors (big and small pharma), government/cooperative group sponsors, regulatory agencies, patient advocacy organizations and contract research organizations (CROs), and all of these need to work together with the patient as the center of the clinical trial universe (Fig. 3 ). Moreover, this whole system needs a digital overhaul as many sites still use protocol binders, pen-and-paper diaries, faxes between sites, unstructured data and decades-old software systems. Registrational clinical trials need to be well managed on a day-to-day basis with rigorous electronic data capture and monitoring. Integration of blockchain technology into the clinical trial management system could conceivably bolster trust in the clinical trial process and facilitate regulatory oversight 40 .
Patient participation in clinical trials is key, as there can be no trials without patients. Clinical trial organizers should make it easier for patients to participate in trials. In addition, physician–patient treatment decisions for major diseases should include clinical trial options as standard. These clinical trials should be easily accessible and should ensure that no patients are unnecessarily excluded; this can be achieved with site-agnostic clinical trial matching and navigation services. In addition, clinical trial training should be a part of medical education so that a diverse pool of trained investigators and personnel from the entire healthcare enterprise can be available for clinical research.
It is about time
Clinical development timelines for drug candidates are a race against time from when patents are filed to final FDA approval 41 . Drug development timelines, on average, are approximately 10 years (Fig. 1 ). The swiftness of the development of the COVID-19 mRNA vaccines and the oral COVID-19 treatment nirmatrelvir/ritonavir tablets, both of which were developed within a year using a ‘lightspeed approach’, should not be an outlier 42 . The lessons learned should provide a model for multiple therapeutic areas of unmet need. The two small molecules that hold the record for the shortest timeline in drug development, osimertinib for EGFR -mutant non-small-cell lung cancer (NSCLC) (984 days via accelerated approval) and elexacaftor for cystic fibrosis (1,043 days via the regular path) 41 , in nonpandemic times demonstrate that this is possible.
The regulatory logjams slowing drug development necessitated the creation of programs such as the FDA’s accelerated approval pathway, which was introduced in 1992 to address the HIV and AIDS crisis and has since benefitted highly specialized areas such as precision oncology 43 . Multiple programs have been created to shorten timelines for the premarket process, including priority review, fast-track designation, breakthrough designation and orphan designation 44 . Beyond these programs, however, the timelines are still slow and there is an urgent need to address this for all diseases as drug development speed is crucial for patients, physicians and drug development stakeholders alike.
Globalizing drug development, harmonization and transportability
Although the mandate of the FDA is to the US population, their influence is global and, functionally, the FDA is the de facto regulator for the world. Other regulatory authorities such as the European Medicines Agency, the National Medical Products Administration in China and the Central Drugs Standard Control Organization in India, which in total serve more than 3 billion of the world’s population, are also evolving as key players in the global pharmaceutical sector. In addition, the newly established African Medicines Agency was set up (in 2019) to speed up timelines for vaccines and medicine approvals and to improve access to drugs, especially for emerging infectious diseases endemic to the continent 45 . All of these agencies need to be able to stand alone. In addition, there is an urgent need for global harmonization across regulatory authorities to address the substantial inequities in access to medicines. Ideally, clinical trials for new therapies should be conducted globally, for access and generalizability 46 . However, the reality is that clinical trials, including RCTs, cannot be conducted in every country to generate specific evidence for that country’s population. Evidence generation using transportability analysis is gaining traction and refers to the ability to generalize inferences from a study sample in one country to a target population in another country where the study was not conducted 47 , 48 . Transportability analyses may offer some evidence of external validity with implications for local regulatory and health technology assessments 48 .
Evidence generation in clinical research
Clinical studies of rare diseases.
As scientific advances drive clinical trials forward, trials on cancers and many rare diseases are being designed and conducted in small genetically defined or biomarker-defined subsets. Moreover, new methods to generate evidence of clinical benefit may accelerate clinical trial conduct and provide individuals with rare diseases access to new therapeutic compounds. Rare diseases affect an estimated 263 million–446 million people globally at any given time and are increasingly becoming a huge public health burden 49 . Clinical trials in this context come with their own challenges stemming from the rarity of the conditions and incomplete natural history data 50 . However, remarkable advances in molecular biology coupled with legislation to spur orphan disease developmental therapeutics have led to progress. There is increasing regulatory flexibility to use programs such as the accelerated approval program, and there are case scenarios whereby trials have used external control arms based on RWD.
As an example, the FDA granted accelerated approval to alpelisib (Vijoice, Novartis) for adults and children over 2 years of age who require systemic therapy for PIK3CA-related overgrowth spectrum, which includes a group of rare disorders linked to mutations in the PIK3CA gene 51 . Interestingly, efficacy was evaluated using a retrospective chart review of RWD from EPIK-P1 ( NCT04285723 ), a single-arm clinical study in which individuals with PIK3CA-related overgrowth spectrum received alpelisib as part of an expanded access program for compassionate use. The application for this approval used the Real-Time Oncology Review pilot program 52 , which streamlined data submission before filing of the entire clinical application, and Assessment Aid 53 , a voluntary submission from the applicant to facilitate assessment by the FDA. As a result, this application was granted priority review, breakthrough designation and orphan drug designation 51 .
N-of-1 trials
In the era of individualized genomic medicine, N-of-1 trials are emerging as a tool to study potentially fatal rare diseases. The N-of-1 trial is a single-patient clinical trial using the individual person as a unit of investigation to evaluate the efficacy and/or adverse events of different interventions through objective data-driven criteria 54 . For example, an antisense oligonucleotide therapy was designed for, and evaluated in, a single patient who had a fatal genetic neurodegenerative disorder known as CLN7 neuronal ceroid lipofuscinosis (a form of Batten’s disease) 55 . Another patient (who happened to be a physician) with idiopathic Castleman’s disease refractory to IL-6-blocking therapy identified the causative molecular alteration in his own disease to develop a personalized therapy 56 . In yet another example, rapid dose escalation with a selective RET inhibitor was evaluated in a single patient with highly refractory medullary thyroid carcinoma, to overcome a resistance mechanism specific to that patient 57 .
These sensational new drug discovery–translation paradigms raise important questions, such as what level of evidence is needed before exposing a human to a new drug, what evidence this approach might generate for the next patient and what challenges might exist with generalizability 58 . The concept of medical analog patient-specific ‘digital twins’ is an emerging area of research that has the potential to combine polynomial data (mechanistic data, medical history, with the power of AI) and may perhaps serve to enhance N-of-1 trials in the future, to further personalize medicine 37 , 59 , 60 .
RWD and real-world evidence
One of the major criticisms of all clinical trial research is that clinical trials do not represent the ‘real-world’ population; often, the restrictive criteria of clinical trials and the limited analyses framed to answer specific questions may not apply to real-world patients. A wide gap therefore exists between the trial world and the real world, and attempts have been made to close this gap 61 . Conventional trials have been designed on the basis of the misconception that regulatory bodies may not accommodate more modern and diverse evidence from RWD, which is no longer the case 61 , 62 .
It is important to distinguish between RWD, which refers to data generated from routine, standard care of patients 62 , and real-world ‘evidence’ (RWE), which is the evidence generated from RWD regarding the potential use of a product. RWE is generated by trial designs or analysis and is not restricted to randomized trials; instead, it comes from pragmatic trials and prospective and/or retrospective observational studies 62 , 63 .
In this purview of RWD and RWE, all stakeholders look to regulators for guidance. Consequently, regulators have taken a hands-on approach and provided guidance and a comprehensive framework launched through the 21st Century Cures Act 62 , 64 . Moreover, the FDA uses RWD and RWE for postmarketing safety monitoring, and insurance agencies have started to use such data for coverage decisions 62 . This has been necessitated by rapidly accelerating data input from multiple streams and layers into electronic health records, as well as wearables and biosensors, in parallel with new analytical capabilities (multimodal AI) to analyze the vast amount of data.
Evidence from synthetic or external control arms
RCTs are considered the gold standard for drug development and evidence as they allow for estimation of treatment effects that can be assigned to the experimental arm of interest. The randomization in these studies curtails the concern for confounding bias by removing systematic imbalances between arms in measured and unmeasured prognostic factors 65 . However, advances in the genomics of rare diseases and the discovery of rare oncogene-driven cancers have led to specific targeted therapies, for which evaluation in RCTs may not be feasible or ethical and may delay patient access to promising or lifesaving therapies.
In such cases, synthetic control arms are emerging as options for generating comparator arms that can ‘mimic’ the comparator arms of RCTs. Synthetic control arms are external to the study in question, and most are derived from RWD 65 . Moreover, RWD are obtained from electronic health records, administrative claims data, natural history registries and patient-generated data from many sources, including wearable devices 65 . Synthetic control arms may also be generated from previous clinical trial data (single or pooled trials). This is an emerging area primed for innovation as so much data are now available from multiple sources.
NSCLC is increasingly being divided into small oncogene-driven subsets, making it more challenging to conduct randomized trials 66 , and recent developments in the NSCLC trial landscape illustrate the utility of synthetic control arms. For instance, RET fusions are genomic drivers in 1–2% of NSCLCs, and pralsetinib is a selective RET-targeted therapy showing promising responses even in individuals with advanced disease. The ARROW study ( NCT03037385 ) was a single-arm registrational trial, conducted globally, to evaluate pralsetinib in RET fusion-positive individuals with NSCLC 67 , 68 . This trial showed a relative survival benefit with the drug when compared to an external standard-of-care control arm consisting of RWD cohorts derived from two Flatiron Health databases 66 . A template for future studies of this nature using quantitative bias analyses showed that comparisons between the external control arm and the trial arm are robust and able to withstand issues such as data missingness, potentially poorer outcomes in RWD and residual confounding 66 . Overall, the study provided evidence in favor of pralsetinib as a first-line treatment for RET fusion-positive NSCLC.
The use of synthetic control arms can accelerate drug development, and initial skepticism about them arose mainly from a lack of precedence and direction from regulatory authorities. These concerns are now being dispelled as synthetic control arms have been used recently for drug approvals for ultra-rare diseases. For example, neurofibromatosis is a rare disease seen in 1 in 3,000 births. Patients develop plexiform neurofibroma lesions that are painful and debilitating, causing motor and neuronal dysfunction. The MEK inhibitor selumetinib was approved for pediatric patients with symptomatic, inoperable plexiform neurofibromas on the basis of a dataset of 50 patients from Selumetinib in Pediatric Neurofibroma Trial (SPRINT)—a single-arm phase 2 trial showing a durable objective response rate and improvements in functional symptoms 65 , 69 , 70 . Comparator arms from two previously conducted trials provided evidence for the natural history of the disease and were submitted as an external control arm, which helped confirm that spontaneous regressions were uncommon and that the observed responses and symptom improvement represented a genuine treatment effect 69 .
Despite this progress, external control arms are still an emerging concept and they have mainly been used to investigate the natural history of disease and have not generally been included as primary evidence or in product labels. However, in the future, I can envision such comparative effectiveness analysis and comparator arms as primary evidence to support drug approval. Challenges mainly arise from data quality and data missingness, as well as uncertainty of whether external control data are fit for purpose. However, some of these concerns can be mitigated by quantitative bias analysis and other methodologies 66 , 71 .
Pediatric clinical trials
Although pediatric research has been at the forefront of major advances in medicine (extracorporeal membrane oxygenation 72 is a notable example) and has pushed the boundaries of modern oncology (for instance, in treating pediatric leukemia), innovations in new drug development are often delayed. Many rare and orphan diseases occur mainly in the pediatric population, and drug development in this population has always been operationally, ethically, statistically and methodologically challenging 73 , 74 . This is compounded by the limited understanding of basic biology, the ontology of disease manifestations, and the acute and long-term safety of products 73 , 74 . In addition, there is considerable off-label use of products in very young children, infants and neonates where clinical trials have not been feasible, and it is imperative that high-level evidence be generated by creative methods. Programs such as the Best Pharmaceuticals for Children Act (in 2002) and the Pediatric Research Equity Act (in 2003), made permanent in 2012 under the FDA Safety and Innovation Act, have incentivized and enhanced the development of pediatric therapeutics 73 . Innovative trial designs, RWD and leveraging data from other resources may help with risk–benefit assessment and drug approval, such as the approval for neurofibromatosis type 1 (NF1) 73 .
Reimagining the future of clinical trials
The landscape of AI in medicine has transformed recently, and AI is poised to become ubiquitous. Several RCTs have quantified the benefits of AI in specialties that use pattern recognition and interpretation of images, such as radiology (mammography and lung cancer screening), cardiology (interpreting electrocardiograms (EKGs), cardiac functional assessment and atrial fibrillation screening), gastroenterology (interpreting colonoscopies), pathology (cancer diagnosis), neurology (tracking disease evolution of amyotrophic lateral sclerosis and Parkinson’s disease), dermatology (diagnosing lesions) and ophthalmology (eye disease screening) 75 . However, most AI research focuses on ‘clinical care delivery’ applications and not ‘clinical trial research’ 76 .
The integration of AI into clinical trial research has been slower than expected, mainly owing to the (perceived) friction between AI versus human intelligence. Nevertheless, trials of data generation and interpretation should be conducted, and AI should be used to augment human intelligence—not seen as something to replace it 77 . Next-generation clinical trials using AI should consider AI + human rather than AI versus human scenarios 75 , 78 . The clinical trial guidelines for protocols (Standard Protocol Items: Recommendations for Interventional Trials–Artificial Intelligence (SPIRIT-AI) extension) and publications (Consolidated Standards of Reporting Trials–Artificial Intelligence (CONSORT-AI) extension) 79 , 80 are intended to achieve standardized and transparent reporting for randomized clinical trials involving AI, and these are just the beginning of a new phase of clinical research modernization.
Given the time and cost involved in developing a drug, every failed drug in the market represents a considerable loss to the drug development ecosystem. In addition, inferior trial designs, suboptimal patient recruitment, poor infrastructure to run trials, and inefficiency in trial conduct and monitoring have plagued the system for decades. AI has the potential to augment all phases of drug development, from drug design to the complete drug development cycle (Fig. 1 ).
Clinical trial conduct is still rudimentary in many ways. For instance, in oncology trials, a few aspects of two-dimensional lesions are measured and followed over time and effectiveness of the drug is evaluated by shrinkage of these lesions. Automated quantitative assessments and artificial neural networks can aid in automated rapid processing of multiple lesions 81 . In cardiology trials, vital signs are measured once a week in clinic, and, in neurology, patient questionnaires are administered in clinic. Now, these data can all be tracked dynamically in real time using wearable sensor technology. The application of AI to such areas can have a transformational near-term impact. In addition, pattern recognition using deep neural networks can help with reading scans, pathology images and EKGs, among others 37 , 78 .
The current evidence-based medicine pyramid represents the tip of the iceberg and barely provides shallow evidence to care for a generic patient (Fig. 4 ). Hence, a deep synthesis and amalgamation of all available data is needed to achieve next-generation, ‘deep’ evidence-based medicine. The main challenge in the next two decades will be to tap the potential of multidimensional evidence generation 82 by extracting, collating and mining large sets of natural history data, genomics and all other omics analysis, all published clinical studies, RWD, data from ubiquitous smart devices and amassed data from the IoMT to provide next-generation evidence for deep medicine.
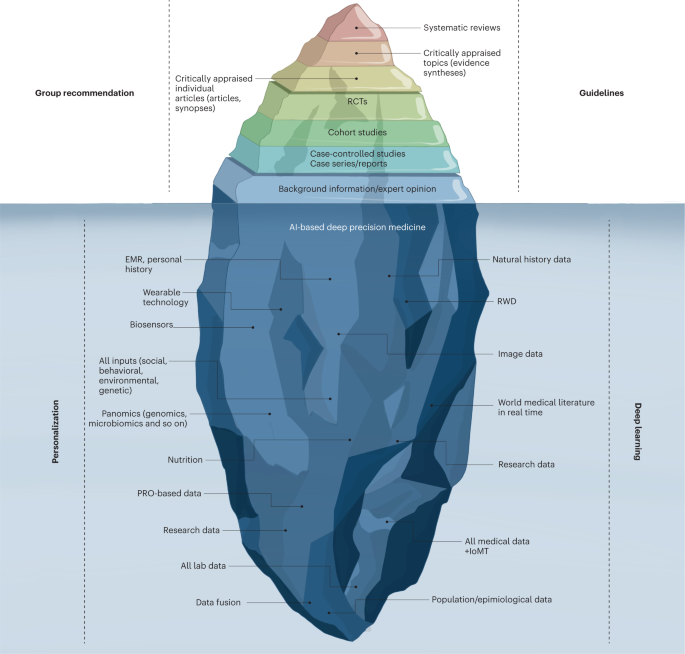
The current evidence-based medicine (EBM) pyramid represents the tip of the iceberg and barely provides enough shallow evidence to care for a generic patient. Hence, a deep synthesis and amalgamation of all available data is needed to achieve next-generation, deep evidence-based medicine. The main challenge ahead in the next two decades will be extracting, collating and mining large sets of natural history data, genomics and all omics analyses, all published clinical studies, RWD and amassed data from the IoMT to provide next-generation evidence for deep medicine. PRO, patient-reported outcomes.
Partnerships in drug development
Currently, the pharma industry is the main driver of drug development, and their expenditures far exceed investments from any national agency such as the National Institutes of Health 61 . There are two domains of clinical trials. The first of these is from ‘big pharma’, which uses CROs to run trials; such trials are very often approved for registration by the FDA. The second domain encompasses academic clinical trials, which often operate on a very limited budget, do not often evaluate new compounds and, thus, rarely result in FDA registration. In this era of reduced federal funding for research, more partnerships are needed for drug development. Academic centers and community sites are crucial for patient enrollment; however, a siloed mentality has impacted drug development and delayed access to lifesaving therapies. Therefore, collaborations among specific disease organizations, academic institutions, federal agencies and patient advocacy groups are crucial for betterment of the health of populations (Fig. 3 ). Because the pharma industry is hesitant to invest huge amounts with limited financial return, especially in rare diseases, federal agencies have developed programs to incentivize rare disease drug development 1 . Moreover, disease-focused organizations have collaborated with the pharma industry, federal agencies and academia to form ‘venture philanthropy’ with risk-sharing financial models to de-risk drug development 1 . Many academic institutions are entering into risk-sharing strategic alliances with the pharma industry to collaborate across preclinical and clinical development phases. Such successful innovative partnership models have set a precedent in diseases such as cystic fibrosis, multiple myeloma, type 1 diabetes mellitus and other rare diseases 1 . These collaborations have effectively catalyzed innovation through all phases of drug development and provided a compelling reason to sustain and foster more of these sorts of programs.
Social media and online community research
Social media outlets (Twitter, Facebook and so on) can influence patient accrual in clinical trials. They can strongly influence and address historical clinical trial challenges, including the lack of awareness among patients and physicians about available trials and the lack of community engagement. More than 4.48 billion people use social media globally, and this number is projected to increase to almost 6 billion in 2027 (ref. 83 ). Over 70% of Americans are on social media, including rural dwellers and adolescent and young adult populations who have always been under-represented in clinical trials. Although many older adults do not use social media, their caregivers are likely to.
People with terminal diseases often self-experiment with drugs, and online patient communities can provide environments for sharing and monitoring such drug usage. This can allow for observational studies to be planned around quantitative, internet-based outcome data. For example, researchers developed an algorithm to dissect the data reported on the PatientsLikeMe website by people with amyotrophic lateral sclerosis who experimented with lithium carbonate treatment 84 . This analysis reached the same conclusion as an ensuing RCT, suggesting that data from online patient behavior can help accelerate drug development and evaluate the effectiveness of drugs already in use.
An increase in engagement from patients and patient advocacy groups can aid patient education and outreach and can facilitate patient-partnered research, as well as allowing for incorporation of patients’ perspectives in the design of clinical research—ultimately generating research that is driven by the needs of real people with the disease under investigation. Moreover, social media breaks open silos dividing researchers and clinicians, creating enormous potential to influence all areas of medicine 85 .
The success of future clinical trials requires a fundamental transformation in how trials are designed, conducted, monitored, adapted, reported and regulated to generate the best evidence. The status quo model is unsustainable. Instead, preventive, personalized, pragmatic and patient-participatory medicine is needed, and paradigm shifts are required to get there via sustainable growth. Silos need to be broken. Standards of care and clinical trials are currently viewed in different realms; however, the overarching goal of both is to improve health outcomes. The COVID-19 pandemic created an opportunity to observe how routine clinical care and clinical trials can work synergistically to generate evidence 86 . Pragmatic platform trials such as the RECOVERY trial should be a model and guide for trial efficiency and real-time impact.
Current paradigms must be continuously challenged by emerging technology and by all stakeholders (the new generations of scientists, physicians, the pharma industry, regulatory authorities and, most importantly, patients). Disruptive innovation should lead to every clinical site being a research site, with all necessary quality checks and research as part of the standard of care. The healthcare system should be integrated into an intuitive RWE-generation system, with clinical research and clinical care going hand in hand. Beyond an ad hoc creative flash of genius (necessitated by a pandemic), sustained momentum will be needed to leverage the knowledge gained from programs such as ‘Operation Warp Speed’ (initiated by the US government to accelerate COVID-19 vaccine development). My personal view is that every major disease needs a ‘Moonshot’ program and every rare disease should have an ‘Operation Warp Speed’—both with clearly identified, sustainable goals to improve population health and address equity, diversity and global access to therapies. Methodological advances and future AI-based analyses of all data will provide deep evidence to realize the goal of personalized medicine— that is, to offer the right treatment to the right patient at the right time.
Ramsey, B. W., Nepom, G. T. & Lonial, S. Academic, foundation, and industry collaboration in finding new therapies. N. Engl. J. Med. 376 , 1762–1769 (2017).
Article CAS Google Scholar
Butler, D. Translational research: crossing the valley of death. Nature 453 , 840–842 (2008).
DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47 , 20–33 (2016).
Article Google Scholar
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 323 , 844–853 (2020).
Subbiah, V. A global effort to understand the riddles of COVID-19 and cancer. Nat. Cancer 1 , 943–945 (2020).
Flaherty, K. T. et al. Rethinking cancer clinical trial conduct induced by COVID-19: an academic center, industry, government, and regulatory agency perspective. Cancer Discov. 11 , 1881–1885 (2021).
Samimi, G. et al. Lessons learned from the impact of COVID-19 on NCI-sponsored cancer prevention clinical trials: moving toward participant-centric study designs. Cancer Prev. Res. 15 , 279–284 (2022).
National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Health and Economic Costs of Chronic Diseases https://www.cdc.gov/chronicdisease/programs-impact/pop/index.htm (2022).
Menta, A. K., Subbiah, I. M. & Subbiah, V. Bringing wearable devices into oncology practice: fitting smart technology in the clinic. Discov. Med. 26 , 261–270 (2018).
Google Scholar
Krittanawong, C., Johnson, K. W. & Tang, W. W. How artificial intelligence could redefine clinical trials in cardiovascular medicine: lessons learned from oncology. Per. Med. 16 , 83–88 (2019).
Subbiah, V. & Kurzrock, R. Challenging standard-of-care paradigms in the precision oncology era. Trends Cancer 4 , 101–109 (2018).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377 , 62–70 (2017).
Park, J. J. H. et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials 20 , 572 (2019).
Dickson, D. et al. The master observational trial: a new class of master protocol to advance precision medicine. Cell 180 , 9–14 (2020).
Das, S. & Lo, A. W. Re-inventing drug development: a case study of the I-SPY 2 breast cancer clinical trials program. Contemp. Clin. Trials 62 , 168–174 (2017).
Redman, M. W. et al. Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol. Lancet Oncol. 21 , 1589–1601 (2020).
Subbiah, V. et al. Pan-cancer efficacy of vemurafenib in BRAF V600 -mutant non-melanoma cancers. Cancer Discov. 10 , 657–663 (2020).
Subbiah, V. et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600 -mutant anaplastic thyroid cancer. J. Clin. Oncol. 36 , 7–13 (2018).
Subbiah, V. et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 28 , 1640–1645 (2022).
Drilon, A. et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N. Engl. J. Med. 383 , 813–824 (2020).
Subbiah, V. et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 23 , 1261–1273 (2022).
Normand, S.-L. T. The RECOVERY platform. N. Engl. J. Med. 384 , 757–758 (2020).
Turner, N. C. et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 21 , 1296–1308 (2020).
Gold, S. M. et al. Platform trials and the future of evaluating therapeutic behavioural interventions. Nat. Rev. Psychol. 1 , 7–8 (2022).
Dickson, D. et al. Snapshot: trial types in precision medicine. Cell 181 , 208 (2020).
Ochoa, D. et al. Human genetics evidence supports two-thirds of the 2021 FDA-approved drugs. Nat. Rev. Drug Discov. 21 , 551 (2022).
Wickström, K. & Moseley, J. Biomarkers and surrogate endpoints in drug development: a european regulatory view. Invest. Ophthalmol. Vis. Sci. 58 , BIO27–BIO33 (2017).
Robb, M. A., McInnes, P. M. & Califf, R. M. Biomarkers and surrogate endpoints: developing common terminology and definitions. JAMA 315 , 1107–1108 (2016).
Landers, M., Dorsey, R. & Saria, S. Digital endpoints: definition, benefits, and current barriers in accelerating development and adoption. Digit. Biomark. 5 , 216–223 (2021).
Yang, Y. et al. Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat. Med. 28 , 2207–2215 (2022).
Perez, M. V. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 381 , 1909–1917 (2019).
Guo, Y. et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J. Am. Coll. Cardiol. 74 , 2365–2375 (2019).
Lubitz, S. A. et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit Heart Study. Am. Heart J. 238 , 16–26 (2021).
Rizas, K. D. et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat. Med. 28 , 1823–1830 (2022).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource https://www.ncbi.nlm.nih.gov/books/NBK326791/ (2016).
Desai, A. & Subbiah, V. COVID-19 pandemic and cancer clinical trial pandemonium: finding the silver lining. J. Immunother. Precis. Oncol. 4 , 64–66 (2020).
Acosta, J. N., Falcone, G. J., Rajpurkar, P. & Topol, E. J. Multimodal biomedical AI. Nat. Med. 28 , 1773–1784 (2022).
Food and Drug Administration. Digital health technologies for remote data acquisition in clinical investigations, draft guidance for industry, investigators, and other stakeholders. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/digital-health-technologies-remote-data-acquisition-clinical-investigations (2022).
Thomas, K. A. & Kidziński, Ł. Artificial intelligence can improve patients’ experience in decentralized clinical trials. Nat. Med. https://doi.org/10.1038/s41591-022-02034-4 (2022).
Wong, D. R., Bhattacharya, S. & Butte, A. J. Prototype of running clinical trials in an untrustworthy environment using blockchain. Nat. Commun. 10 , 917 (2019).
Brown, D. G., Wobst, H. J., Kapoor, A., Kenna, L. A. & Southall, N. Clinical development times for innovative drugs. Nat. Rev. Drug Discov. 21 , 793–794 (2021).
Anderson, A. S. A lightspeed approach to pandemic drug development. Nat. Med. 28 , 1538 (2022).
Subbiah, V. et al. Accelerated approvals hit the target in precision oncology. Nat. Med. 28 , 1976–1979 (2022).
Kepplinger, E. E. FDA’s expedited approval mechanisms for new drug products. Biotechnol. Law Rep. 34 , 15–37 (2015).
Ncube, B. M., Dube, A. & Ward, K. Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J. Pharm. Policy Pract. 14 , 29 (2021).
Moyers, J. T. & Subbiah, V. Think globally, act locally: globalizing precision oncology. Cancer Discov. 12 , 886–888 (2022).
Degtiar, I. & Rose, S. A review of generalizability and transportability. Annu. Rev. Stat. Appl. 10 , 1 (2023).
Ramagopalan, S. V. et al. Transportability of overall survival estimates from US to Canadian patients with advanced non–small cell lung cancer with implications for regulatory and health technology assessment. JAMA Netw. Open 5 , e2239874 (2022).
Nguengang Wakap, S. et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur. J. Hum. Genet. 28 , 165–173 (2020).
Pizzamiglio, C., Vernon, H. J., Hanna, M. G. & Pitceathly, R. D. S. Designing clinical trials for rare diseases: unique challenges and opportunities. Nat. Rev. Methods Primers 2 , 13 (2022).
Food and Drug Administration. FDA approves alpelisib for PIK3CA-related overgrowth spectrum. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alpelisib-pik3ca-related-overgrowth-spectrum (2022).
Food and Drug Administration. Real-time oncology review. https://www.fda.gov/about-fda/oncology-center-excellence/real-time-oncology-review (2022).
Food and Drug Administration. Assessment aid. https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid (2022).
Lillie, E. O. et al. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per. Med. 8 , 161–173 (2011).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381 , 1644–1652 (2019).
Fajgenbaum, D. C. et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6 blockade–refractory idiopathic multicentric Castleman disease. J. Clin. Investig. 129 , 4451–4463 (2019).
Subbiah, V. et al. Selective RET kinase inhibition for patients with RET -altered cancers. Ann. Oncol. 29 , 1869–1876 (2018).
Woodcock, J. & Marks, P. Drug regulation in the era of individualized therapies. N. Engl. J. Med. 381 , 1678–1680 (2019).
Björnsson, B. et al. Digital twins to personalize medicine. Genome Med. 12 , 4 (2019).
Laubenbacher, R., Sluka, J. P. & Glazier, J. A. Using digital twins in viral infection. Science 371 , 1105–1106 (2021).
Sherman, R. E., Davies, K. M., Robb, M. A., Hunter, N. L. & Califf, R. M. Accelerating development of scientific evidence for medical products within the existing US regulatory framework. Nat. Rev. Drug Discov. 16 , 297–298 (2017).
Concato, J. & Corrigan-Curay, J. Real-world evidence—where are we now? N. Engl. J. Med. 386 , 1680–1682 (2022).
Concato, J., Stein, P., Dal Pan, G. J., Ball, R. & Corrigan-Curay, J. Randomized, observational, interventional, and real-world—what’s in a name? Pharmacoepidemiol. Drug Saf. 29 , 1514–1517 (2020).
Food and Drug Administration. Real-world evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (2022).
Mishra-Kalyani, P. S. et al. External control arms in oncology: current use and future directions. Ann. Oncol. 33 , 376–383 (2022).
Popat, S. et al. Addressing challenges with real-world synthetic control arms to demonstrate the comparative effectiveness of pralsetinib in non-small cell lung cancer. Nat. Commun. 13 , 3500 (2022).
Gainor, J. F. et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 22 , 959–969 (2021).
Subbiah, V. et al. Pralsetinib for patients with advanced or metastatic RET -altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 9 , 491–501 (2021).
Center for Drug Evaluation and Research, Food and Drug Administration. NDA multi-disciplinary review and evaluation: NDA 213756 Koselugo (selumetinib). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213756Orig1s000MultidisciplineR.pdf (2020).
Casey, D. et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin. Cancer Res. 27 , 4142–4146 (2021).
Wilkinson, S. et al. Assessment of alectinib vs ceritinib in ALK-positive non–small cell lung cancer in phase 2 trials and in real-world data. JAMA Netw. Open 4 , e2126306 (2021).
Bartlett, R. H. et al. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure. 100 cases. Ann. Surg. 204 , 236–245 (1986).
McCune, S. & Portman, R. J. Innovation and opportunities in pediatric therapeutic development. Ther. Innov. Regul. Sci. 53 , 564–566 (2019).
Subbiah, V. Fast-tracking novel drugs in pediatric oncology. Cell Cycle 14 , 1127–1128 (2015).
Rajpurkar, P., Chen, E., Banerjee, O. & Topol, E. J. AI in health and medicine. Nat. Med. 28 , 31–38 (2022).
Weissler, E. H. et al. The role of machine learning in clinical research: transforming the future of evidence generation. Trials 22 , 537 (2021).
Adashek, J. J., Subbiah, I. M. & Subbiah, V. Artificial intelligence systems assisting oncologists? Resist and desist or enlist and coexist. Oncologist 24 , 1291–1293 (2019).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25 , 44–56 (2019).
Liu, X. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26 , 1364–1374 (2020).
Cruz Rivera, S. et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat. Med. 26 , 1351–1363 (2020).
Kickingereder, P. et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 20 , 728–740 (2019).
Jarow, J. P., LaVange, L. & Woodcock, J. Multidimensional evidence generation and FDA regulatory decision making: defining and using “real-world” data. JAMA 318 , 703–704 (2017).
Dean, B. Social network usage & growth statistics: how many people use social media in 2022? Backlinko https://backlinko.com/social-media-users (2021).
Wicks, P., Vaughan, T. E., Massagli, M. P. & Heywood, J. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat. Biotechnol. 29 , 411–414 (2011).
Morgan, G. et al. The (r)evolution of social media in oncology: engage, enlighten, and encourage. Cancer Discov. 12 , 1620–1624 (2022).
Pessoa-Amorim, G. et al. Making trials part of good clinical care: lessons from the RECOVERY trial. Future Healthc. J. 8 , e243–e250 (2021).
Download references
Acknowledgements
V.S. is an Andrew Sabin Family Foundation fellow at the University of Texas MD Anderson Cancer Center. V.S. acknowledges the support of the Jacquelyn A. Brady Fund. V.S. thanks the team at Draw Impacts for figures. V.S. is supported by the US National Institutes of Health (NIH) (grants R01CA242845 and R01CA273168); the MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (grant RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (grant 1U01CA180964), NCATS (Center for Clinical and Translational Sciences) (grant UL1TR000371) and the MD Anderson Cancer Center Support (grant P30CA016672).
Author information
Authors and affiliations.
Department of Investigational Cancer Therapeutics, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Vivek Subbiah
Division of Pediatrics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
MD Anderson Cancer Network, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Vivek Subbiah .
Ethics declarations
Competing interests.
None relevant to the manuscript. V.S. reports research funding/grant support for clinical trials from AbbVie, Agensys, Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines, Boston Biomedical, Boston Pharmaceuticals, Celgene, D3 Bio, Dragonfly Therapeutics, Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Incyte, Inhibrx, Loxo Oncology, MedImmune, MultiVir, NanoCarrier, National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center and Vegenics; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte, Novartis and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo and R-Pharm US; and a relationship with Medscape.
Peer review
Peer review information.
Nature Medicine thanks Benedikt Westphalen, Edward Mills and Christina Yap for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Subbiah, V. The next generation of evidence-based medicine. Nat Med 29 , 49–58 (2023). https://doi.org/10.1038/s41591-022-02160-z
Download citation
Received : 28 September 2022
Accepted : 11 November 2022
Published : 16 January 2023
Issue Date : January 2023
DOI : https://doi.org/10.1038/s41591-022-02160-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Tribulations and future opportunities for artificial intelligence in precision medicine.
- Claudio Carini
- Attila A. Seyhan
Journal of Translational Medicine (2024)
Mapping the landscape of research on insulin resistance: a visualization analysis of randomized clinical trials
- Sa’ed H. Zyoud
Journal of Health, Population and Nutrition (2024)
Nurse managers’ managerial innovation and it’s relation to proactivity behavior and locus of control among intensive care nurses
- Loly Mohamed Shawky Elbus
- Mohamed Gamal Mostafa
- Seham Aly Mahmoud
BMC Nursing (2024)
Pharmacological reactivation of p53 in the era of precision anticancer medicine
- Charlotte Strandgren
- Klas G. Wiman
Nature Reviews Clinical Oncology (2024)
A guide to artificial intelligence for cancer researchers
- Raquel Perez-Lopez
- Narmin Ghaffari Laleh
- Jakob Nikolas Kather
Nature Reviews Cancer (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Performing a...
Performing a literature review
- Related content
- Peer review
- Gulraj S Matharu , academic foundation doctor ,
- Christopher D Buckley , Arthritis Research UK professor of rheumatology
- 1 Institute of Biomedical Research, College of Medical and Dental Sciences, School of Immunity and Infection, University of Birmingham, UK
A necessary skill for any doctor
What causes disease, which drug is best, does this patient need surgery, and what is the prognosis? Although experience helps in answering these questions, ultimately they are best answered by evidence based medicine. But how do you assess the evidence? As a medical student, and throughout your career as a doctor, critical appraisal of published literature is an important skill to develop and refine. At medical school you will repeatedly appraise published literature and write literature reviews. These activities are commonly part of a special study module, research project for an intercalated degree, or another type of essay based assignment.
Formulating a question
Literature reviews are most commonly performed to help answer a particular question. While you are at medical school, there will usually be some choice regarding the area you are going to review.
Once you have identified a subject area for review, the next step is to formulate a specific research question. This is arguably the most important step because a clear question needs to be defined from the outset, which you aim to answer by doing the review. The clearer the question, the more likely it is that the answer will be clear too. It is important to have discussions with your supervisor when formulating a research question as his or her input will be invaluable. The research question must be objective and concise because it is easier to search through the evidence with a clear question. The question also needs to be feasible. What is the point in having a question for which no published evidence exists? Your supervisor’s input will ensure you are not trying to answer an unrealistic question. Finally, is the research question clinically important? There are many research questions that may be answered, but not all of them will …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £50 / $60/ €56 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article

University Libraries
- Ohio University Libraries
- Library Guides
Evidence-based Practice in Healthcare
- Performing a Literature Review
- EBP Tutorials
- Question- PICO
- Definitions
- Systematic Reviews
- Levels of Evidence
- Finding Evidence
- Filter by Study Type
- Too Much or Too Little?
- Critical Appraisal
- Quality Improvement (QI)
- Contact - Need Help?
Hanna's Performing a qualitity literature review presentation slides
- Link to the PPT slides via OneDrive anyone can view
Characteristics of a Good Literature Review in Health & Medicine
Clear Objectives and Research Questions : The review should start with clearly defined objectives and research questions that guide the scope and focus of the review.
Comprehensive Coverage : Include a wide range of relevant sources, such as research articles, review papers, clinical guidelines, and books. Aim for a broad understanding of the topic, covering historical developments and current advancements. To do this, an intentional and minimally biased search strategy.
- Link to relevant databases to consider for a comprehensive search (search 2+ databases)
- Link to the video "Searching your Topic: Strategies and Efficiencies" by Hanna Schmillen
- Link to the worksheet "From topic, to PICO, to search strategy" to help researchers work through their topic into an intentional search strategy by Hanna Schmillen
Transparency and Replicability : The review process, search strategy, should be transparent, with detailed documentation of all steps taken. This allows others to replicate the review or update it in the future.
Appraisal of Studies Included : Each included study should be critically appraised for methodological quality and relevance. Use standardized appraisal tools to assess the risk of bias and the quality of evidence.
- Link to the video " Evaluating Health Research" by Hanna Schmillen
- Link to evaluating and appraising studies tab, which includes a rubric and checklists
Clear Synthesis and Discussion of Findings : The review should provide a thorough discussion of the findings, including any patterns, relationships, or trends identified in the literature. Address the strengths and limitations of the reviewed studies and the review itself. Present findings in a balanced and unbiased manner, avoiding over interpretation or selective reporting of results.
Implications for Practice and Research : The review should highlight the practical implications of the findings for medical practice and policy. It should also identify gaps in the current literature and suggest areas for future research.
Referencing and Citation : Use proper citation practices to credit original sources. Provide a comprehensive reference list to guide readers to the original studies.
- Link to Citation Style Guide, includes tab about Zotero
Note: A literature review is not a systematic review. For more information about systematic reviews and different types of evidence synthesis projects, see the Evidence Synthesis guide .
- << Previous: Quality Improvement (QI)
- Next: Contact - Need Help? >>
- UNC Libraries
- HSL Academic Process
- Evidence-Based Medicine: Review and Practice
- Introduction
Evidence-Based Medicine: Review and Practice: Introduction
Created by health science librarians.

- Listen: What is Evidence-Based Medicine?
- Steps in the EBM process
- Listen: What is the question?
- Test your PICO skills
- How to search for evidence
- Turn your PICO into a search
- Where to search for evidence
- Listen: Study designs and evidence
- Filtering by study design
- Test your evidence ranking skills
- Appraise: Evaluating article content
- Appraisal tools
- Test your quick appraisal skills
- Apply & evaluate
- Assess your EBM skills
- More resources
- How did we do?
Learning Objectives
This module is intended to help you develop your evidence-based medicine (EBM) skills. The goal is to increase your comfort and ability to ask patient care related questions and efficiently find evidence to incorporate into patient management. Like many skills in medicine, EBM takes practice to master.
After a brief review of the definition and process of evidence-based medicine, the primary focus of the module is to guide your practice of turning clinical questions into database searches and selecting the best available evidence from the results to critically appraise and apply.
For more detailed background on EBM see:
Introduction to Evidence-Based Practice Tutorial (UNC & Duke)
Ten essential papers for the practice of evidence-based medicine In this 2017 BMJ Evidence-Based Medicine journal article, the authors signpost readers to 10 papers considered essential reading for anyone starting out on an evidence-based medicine journey.
JAMAevidence Provides tools for students and clinicians to evaluate and interpret medical literature and better understand the principles of Evidence Based Practice/Medicine, including the books Users' Guides to Medical Literature, The Rational Clinical Examination , etc.
Centre for Evidence Based Medicine: EBM Resources
To review basic PubMed techniques useful in EBM searches see:
Searching PubMed Created by HSL clinical librarians, this guide provides a way to quickly review the basic PubMed search skills needed to answer clinical questions.
PubMed Online Training These resources from the National Library of Medicine include tutorials, classes, handouts, and more.
- Next: What is Evidence-Based Medicine? >>
- Last Updated: May 14, 2024 12:49 PM
- URL: https://guides.lib.unc.edu/ebm-review
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 21, Issue 4
- New evidence pyramid
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- M Hassan Murad ,
- Mouaz Alsawas ,
- http://orcid.org/0000-0001-5481-696X Fares Alahdab
- Rochester, Minnesota , USA
- Correspondence to : Dr M Hassan Murad, Evidence-based Practice Center, Mayo Clinic, Rochester, MN 55905, USA; murad.mohammad{at}mayo.edu
https://doi.org/10.1136/ebmed-2016-110401
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- EDUCATION & TRAINING (see Medical Education & Training)
- EPIDEMIOLOGY
- GENERAL MEDICINE (see Internal Medicine)
The first and earliest principle of evidence-based medicine indicated that a hierarchy of evidence exists. Not all evidence is the same. This principle became well known in the early 1990s as practising physicians learnt basic clinical epidemiology skills and started to appraise and apply evidence to their practice. Since evidence was described as a hierarchy, a compelling rationale for a pyramid was made. Evidence-based healthcare practitioners became familiar with this pyramid when reading the literature, applying evidence or teaching students.
Various versions of the evidence pyramid have been described, but all of them focused on showing weaker study designs in the bottom (basic science and case series), followed by case–control and cohort studies in the middle, then randomised controlled trials (RCTs), and at the very top, systematic reviews and meta-analysis. This description is intuitive and likely correct in many instances. The placement of systematic reviews at the top had undergone several alterations in interpretations, but was still thought of as an item in a hierarchy. 1 Most versions of the pyramid clearly represented a hierarchy of internal validity (risk of bias). Some versions incorporated external validity (applicability) in the pyramid by either placing N-1 trials above RCTs (because their results are most applicable to individual patients 2 ) or by separating internal and external validity. 3
Another version (the 6S pyramid) was also developed to describe the sources of evidence that can be used by evidence-based medicine (EBM) practitioners for answering foreground questions, showing a hierarchy ranging from studies, synopses, synthesis, synopses of synthesis, summaries and systems. 4 This hierarchy may imply some sort of increasing validity and applicability although its main purpose is to emphasise that the lower sources of evidence in the hierarchy are least preferred in practice because they require more expertise and time to identify, appraise and apply.
The traditional pyramid was deemed too simplistic at times, thus the importance of leaving room for argument and counterargument for the methodological merit of different designs has been emphasised. 5 Other barriers challenged the placement of systematic reviews and meta-analyses at the top of the pyramid. For instance, heterogeneity (clinical, methodological or statistical) is an inherent limitation of meta-analyses that can be minimised or explained but never eliminated. 6 The methodological intricacies and dilemmas of systematic reviews could potentially result in uncertainty and error. 7 One evaluation of 163 meta-analyses demonstrated that the estimation of treatment outcomes differed substantially depending on the analytical strategy being used. 7 Therefore, we suggest, in this perspective, two visual modifications to the pyramid to illustrate two contemporary methodological principles ( figure 1 ). We provide the rationale and an example for each modification.
- Download figure
- Open in new tab
- Download powerpoint
The proposed new evidence-based medicine pyramid. (A) The traditional pyramid. (B) Revising the pyramid: (1) lines separating the study designs become wavy (Grading of Recommendations Assessment, Development and Evaluation), (2) systematic reviews are ‘chopped off’ the pyramid. (C) The revised pyramid: systematic reviews are a lens through which evidence is viewed (applied).
Rationale for modification 1
In the early 2000s, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group developed a framework in which the certainty in evidence was based on numerous factors and not solely on study design which challenges the pyramid concept. 8 Study design alone appears to be insufficient on its own as a surrogate for risk of bias. Certain methodological limitations of a study, imprecision, inconsistency and indirectness, were factors independent from study design and can affect the quality of evidence derived from any study design. For example, a meta-analysis of RCTs evaluating intensive glycaemic control in non-critically ill hospitalised patients showed a non-significant reduction in mortality (relative risk of 0.95 (95% CI 0.72 to 1.25) 9 ). Allocation concealment and blinding were not adequate in most trials. The quality of this evidence is rated down due to the methodological imitations of the trials and imprecision (wide CI that includes substantial benefit and harm). Hence, despite the fact of having five RCTs, such evidence should not be rated high in any pyramid. The quality of evidence can also be rated up. For example, we are quite certain about the benefits of hip replacement in a patient with disabling hip osteoarthritis. Although not tested in RCTs, the quality of this evidence is rated up despite the study design (non-randomised observational studies). 10
Rationale for modification 2
Another challenge to the notion of having systematic reviews on the top of the evidence pyramid relates to the framework presented in the Journal of the American Medical Association User's Guide on systematic reviews and meta-analysis. The Guide presented a two-step approach in which the credibility of the process of a systematic review is evaluated first (comprehensive literature search, rigorous study selection process, etc). If the systematic review was deemed sufficiently credible, then a second step takes place in which we evaluate the certainty in evidence based on the GRADE approach. 11 In other words, a meta-analysis of well-conducted RCTs at low risk of bias cannot be equated with a meta-analysis of observational studies at higher risk of bias. For example, a meta-analysis of 112 surgical case series showed that in patients with thoracic aortic transection, the mortality rate was significantly lower in patients who underwent endovascular repair, followed by open repair and non-operative management (9%, 19% and 46%, respectively, p<0.01). Clearly, this meta-analysis should not be on top of the pyramid similar to a meta-analysis of RCTs. After all, the evidence remains consistent of non-randomised studies and likely subject to numerous confounders.
Therefore, the second modification to the pyramid is to remove systematic reviews from the top of the pyramid and use them as a lens through which other types of studies should be seen (ie, appraised and applied). The systematic review (the process of selecting the studies) and meta-analysis (the statistical aggregation that produces a single effect size) are tools to consume and apply the evidence by stakeholders.
Implications and limitations
Changing how systematic reviews and meta-analyses are perceived by stakeholders (patients, clinicians and stakeholders) has important implications. For example, the American Heart Association considers evidence derived from meta-analyses to have a level ‘A’ (ie, warrants the most confidence). Re-evaluation of evidence using GRADE shows that level ‘A’ evidence could have been high, moderate, low or of very low quality. 12 The quality of evidence drives the strength of recommendation, which is one of the last translational steps of research, most proximal to patient care.
One of the limitations of all ‘pyramids’ and depictions of evidence hierarchy relates to the underpinning of such schemas. The construct of internal validity may have varying definitions, or be understood differently among evidence consumers. A limitation of considering systematic review and meta-analyses as tools to consume evidence may undermine their role in new discovery (eg, identifying a new side effect that was not demonstrated in individual studies 13 ).
This pyramid can be also used as a teaching tool. EBM teachers can compare it to the existing pyramids to explain how certainty in the evidence (also called quality of evidence) is evaluated. It can be used to teach how evidence-based practitioners can appraise and apply systematic reviews in practice, and to demonstrate the evolution in EBM thinking and the modern understanding of certainty in evidence.
- Leibovici L
- Agoritsas T ,
- Vandvik P ,
- Neumann I , et al
- ↵ Resources for Evidence-Based Practice: The 6S Pyramid. Secondary Resources for Evidence-Based Practice: The 6S Pyramid Feb 18, 2016 4:58 PM. http://hsl.mcmaster.libguides.com/ebm
- Vandenbroucke JP
- Berlin JA ,
- Dechartres A ,
- Altman DG ,
- Trinquart L , et al
- Guyatt GH ,
- Vist GE , et al
- Coburn JA ,
- Coto-Yglesias F , et al
- Sultan S , et al
- Montori VM ,
- Ioannidis JP , et al
- Altayar O ,
- Bennett M , et al
- Nissen SE ,
Contributors MHM conceived the idea and drafted the manuscript. FA helped draft the manuscript and designed the new pyramid. MA and NA helped draft the manuscript.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Editorial Pyramids are guides not rules: the evolution of the evidence pyramid Terrence Shaneyfelt BMJ Evidence-Based Medicine 2016; 21 121-122 Published Online First: 12 Jul 2016. doi: 10.1136/ebmed-2016-110498
- Perspective EBHC pyramid 5.0 for accessing preappraised evidence and guidance Brian S Alper R Brian Haynes BMJ Evidence-Based Medicine 2016; 21 123-125 Published Online First: 20 Jun 2016. doi: 10.1136/ebmed-2016-110447
Read the full text or download the PDF:
The website may be down at times on Saturday, August 24, and Sunday, August 25, for maintenance.

JAY SIWEK, M.D., MARGARET L. GOURLAY, M.D., DAVID C. SLAWSON, M.D., AND ALLEN F. SHAUGHNESSY, PHARM.D.
Am Fam Physician. 2002;65(2):251-258
Traditional clinical review articles, also known as updates, differ from systematic reviews and meta-analyses. Updates selectively review the medical literature while discussing a topic broadly. Nonquantitative systematic reviews comprehensively examine the medical literature, seeking to identify and synthesize all relevant information to formulate the best approach to diagnosis or treatment. Meta-analyses (quantitative systematic reviews) seek to answer a focused clinical question, using rigorous statistical analysis of pooled research studies. This article presents guidelines for writing an evidence-based clinical review article for American Family Physician . First, the topic should be of common interest and relevance to family practice. Include a table of the continuing medical education objectives of the review. State how the literature search was done and include several sources of evidence-based reviews, such as the Cochrane Collaboration, BMJ's Clinical Evidence , or the InfoRetriever Web site. Where possible, use evidence based on clinical outcomes relating to morbidity, mortality, or quality of life, and studies of primary care populations. In articles submitted to American Family Physician , rate the level of evidence for key recommendations according to the following scale: level A (randomized controlled trial [RCT], meta-analysis); level B (other evidence); level C (consensus/expert opinion). Finally, provide a table of key summary points.
American Family Physician is particularly interested in receiving clinical review articles that follow an evidence-based format. Clinical review articles, also known as updates, differ from systematic reviews and meta-analyses in important ways. 1 Updates selectively review the medical literature while discussing a topic broadly. An example of such a topic is, “The diagnosis and treatment of myocardial ischemia.” Systematic reviews comprehensively examine the medical literature, seeking to identify and synthesize all relevant information to formulate the best approach to diagnosis or treatment. Examples are many of the systematic reviews of the Cochrane Collaboration or BMJ's Clinical Evidence compendium. Meta-analyses are a special type of systematic review. They use quantitative methods to analyze the literature and seek to answer a focused clinical question, using rigorous statistical analysis of pooled research studies. An example is, “Do beta blockers reduce mortality following myocardial infarction?”
The best clinical review articles base the discussion on existing systematic reviews and meta-analyses, and incorporate all relevant research findings about the management of a given disorder. Such evidence-based updates provide readers with powerful summaries and sound clinical guidance.
In this article, we present guidelines for writing an evidence-based clinical review article, especially one designed for continuing medical education (CME) and incorporating CME objectives into its format. This article may be read as a companion piece to a previous article and accompanying editorial about reading and evaluating clinical review articles. 1 , 2 Some articles may not be appropriate for an evidence-based format because of the nature of the topic, the slant of the article, a lack of sufficient supporting evidence, or other factors. We encourage authors to review the literature and, wherever possible, rate key points of evidence. This process will help emphasize the summary points of the article and strengthen its teaching value.
Topic Selection
Choose a common clinical problem and avoid topics that are rarities or unusual manifestations of disease or that have curiosity value only. Whenever possible, choose common problems for which there is new information about diagnosis or treatment. Emphasize new information that, if valid, should prompt a change in clinical practice, such as the recent evidence that spironolactone therapy improves survival in patients who have severe congestive heart failure. 3 Similarly, new evidence showing that a standard treatment is no longer helpful, but may be harmful, would also be important to report. For example, patching most traumatic corneal abrasions may actually cause more symptoms and delay healing compared with no patching. 4
Searching the Literature
When searching the literature on your topic, please consult several sources of evidence-based reviews ( Table 1 ) . Look for pertinent guidelines on the diagnosis, treatment, or prevention of the disorder being discussed. Incorporate all high-quality recommendations that are relevant to the topic. When reviewing the first draft, look for all key recommendations about diagnosis and, especially, treatment. Try to ensure that all recommendations are based on the highest level of evidence available. If you are not sure about the source or strength of the recommendation, return to the literature, seeking out the basis for the recommendation.
| The AHRQ Web site includes links to the National Guideline Clearinghouse, Evidence Reports from the AHRQ's 12 Evidence-based Practice Centers (EPC), and Preventive Services. The AHCPR released 19 Clinical Practice Guidelines between 1992 and1996 that were not subsequently updated. | ||
| evaluates evidence in individual articles. Commentary by ACP author offers clinical recommendations. Access to the online version of is a benefit for members of the ACP-ASIM, but will be open to all until at least the end of 2001. | ||
| Features short evaluations/discussions of individual articles dealing with evidence-based clinical practice. | ||
| The University of Oxford/Oxford Radcliffe Hospital Clinical School Web site includes links to CEBM within the Faculty of Medicine, a CATbank (Critically Appraised Topics), links to evidence-based journals, and EBM-related teaching materials. | ||
| The AHRQ began the Translating Research into Practice (TRIP) initiative in 1990 to implement evidence-based tools and information. The TRIP Database features hyperlinks to the largest collection of EBM materials on the internet, including NGC, POEM, DARE, Cochrane Library, CATbank, and individual articles. A good starting place for an EBM literature search. | ||
| , | ||
| Searches BMJ's compendium for up-to-date evidence regarding effective health care. Lists available topics and describes the supporting body of evidence to date (e.g., number of relevant randomized controlled trials published to date). Concludes with interventions “likely to be beneficial” versus those with “unknown effectiveness.” Individuals who have received a free copy of Issue 5 from the United Health Foundation are also entitled to free access to the full online content. | ||
| Systematic evidence reviews that are updated periodically by the Cochrane Group. Reviewers discuss whether adequate data are available for the development of EBM guidelines for diagnosis or management. | ||
| Structured abstracts written by University of York CRD reviewers (see NHS CRD). Abstract summaries review articles on diagnostic or treatment interventions and discuss clinical implications. | ||
| Bi-monthly, peer-reviewed bulletin for medical decision-makers. Based on systematic reviews and synthesis of research on the clinical effectiveness, cost-effectiveness and acceptability of health service interventions. | ||
| Bimonthly publication launched in 1995 by the BMJ Publishing Group. Article summaries include commentaries by clinical experts. Subscription is required. | ||
| Newsletter (including Patient-Oriented Evidence that Matters [POEM])* | ||
| This newsletter features up-to-date POEM, Disease-Oriented Evidence (DOE), and tests approved for Category 1 CME credit. Subscription required. | ||
| Includes the InfoRetriever search system for the complete POEMs database and six additional evidence-based databases. Subscription is required. | ||
| ICSI is an independent, nonprofit collaboration of health care organizations, including the Mayo Clinic, Rochester, Minn. Web site includes the ICSI guidelines for preventive services and disease management. | ||
| Comprehensive database of evidence-based clinical practice guidelines from government agencies and health care organizations. Describes and compares guideline statements with respect to objectives, methods, outcomes, evidence rating scheme, and major recommendations. | ||
| Searches CRD Databases (includes DARE, NHS Economic Evaluation Database, Health Technology Assessment Database) for EBM reviews. More limited than TRIP Database. | ||
| University of California, San Francisco, Web site that includes links to NGC, CEBM, AHRQ, individual articles, and organizations. | ||
| This Web site features updated recommendations for clinical preventive services based on systematic evidence reviews by the U.S. Preventive Services Task Force. | ||
In particular, try to find the answer in an authoritative compendium of evidence-based reviews, or at least try to find a meta-analysis or well-designed randomized controlled trial (RCT) to support it. If none appears to be available, try to cite an authoritative consensus statement or clinical guideline, such as a National Institutes of Health Consensus Development Conference statement or a clinical guideline published by a major medical organization. If no strong evidence exists to support the conventional approach to managing a given clinical situation, point this out in the text, especially for key recommendations. Keep in mind that much of traditional medical practice has not yet undergone rigorous scientific study, and high-quality evidence may not exist to support conventional knowledge or practice.
Patient-Oriented vs. Disease-Oriented Evidence
With regard to types of evidence, Shaughnessy and Slawson 5 – 7 developed the concept of Patient-Oriented Evidence that Matters (POEM), in distinction to Disease-Oriented Evidence (DOE). POEM deals with outcomes of importance to patients, such as changes in morbidity, mortality, or quality of life. DOE deals with surrogate end points, such as changes in laboratory values or other measures of response. Although the results of DOE sometimes parallel the results of POEM, they do not always correspond ( Table 2 ) . 2 When possible, use POEM-type evidence rather than DOE. When DOE is the only guidance available, indicate that key clinical recommendations lack the support of outcomes evidence. Here is an example of how the latter situation might appear in the text: “Although prostate-specific antigen (PSA) testing identifies prostate cancer at an early stage, it has not yet been proved that PSA screening improves patient survival.” (Note: PSA testing is an example of DOE, a surrogate marker for the true outcomes of importance—improved survival, decreased morbidity, and improved quality of life.)
| Antiarrhythmic therapy | Antiarrhythmic drug X decreases the incidence of PVCs on ECGs | Antiarrhythmic drug X is associated with an increase in mortality | POEM results are contrary to DOE implications |
| Antihypertensive therapy | Antihypertensive drug treatment lowers blood pressure | Antihypertensive drug treatment is associated with a decrease in mortality | POEM results are in concordance with DOE implications |
| Screening for prostate cancer | PSA screening detects prostate cancer at an early stage | Whether PSA screening reduces mortality from prostate cancer is currently unknown | Although DOE exists, the important POEM is currently unknown |
Evaluating the Literature
Evaluate the strength and validity of the literature that supports the discussion (see the following section, Levels of Evidence). Look for meta-analyses, high-quality, randomized clinical trials with important outcomes (POEM), or well-designed, nonrandomized clinical trials, clinical cohort studies, or case-controlled studies with consistent findings. In some cases, high-quality, historical, uncontrolled studies are appropriate (e.g., the evidence supporting the efficacy of Papanicolaou smear screening). Avoid anecdotal reports or repeating the hearsay of conventional wisdom, which may not stand up to the scrutiny of scientific study (e.g., prescribing prolonged bed rest for low back pain).
Look for studies that describe patient populations that are likely to be seen in primary care rather than subspecialty referral populations. Shaughnessy and Slawson's guide for writers of clinical review articles includes a section on information and validity traps to avoid. 2
Levels of Evidence
Readers need to know the strength of the evidence supporting the key clinical recommendations on diagnosis and treatment. Many different rating systems of varying complexity and clinical relevance are described in the medical literature. Recently, the third U.S. Preventive Services Task Force (USPSTF) emphasized the importance of rating not only the study type (RCT, cohort study, case-control study, etc.), but also the study quality as measured by internal validity and the quality of the entire body of evidence on a topic. 8
While it is important to appreciate these evolving concepts, we find that a simplified grading system is more useful in AFP . We have adopted the following convention, using an ABC rating scale. Criteria for high-quality studies are discussed in several sources. 8 , 9 See the AFP Web site ( www.aafp.org/afp/authors ) for additional information about levels of evidence and see the accompanying editorial in this issue discussing the potential pitfalls and limitations of any rating system.
Level A (randomized controlled trial/meta-analysis): High-quality randomized controlled trial (RCT) that considers all important outcomes. High-quality meta-analysis (quantitative systematic review) using comprehensive search strategies.
Level B (other evidence): A well-designed, nonrandomized clinical trial. A nonquantitative systematic review with appropriate search strategies and well-substantiated conclusions. Includes lower quality RCTs, clinical cohort studies, and case-controlled studies with non-biased selection of study participants and consistent findings. Other evidence, such as high-quality, historical, uncontrolled studies, or well-designed epidemiologic studies with compelling findings, is also included.
Level C (consensus/expert opinion): Consensus viewpoint or expert opinion.
Each rating is applied to a single reference in the article, not to the entire body of evidence that exists on a topic. Each label should include the letter rating (A, B, C), followed by the specific type of study for that reference. For example, following a level B rating, include one of these descriptors: (1) nonrandomized clinical trial; (2) nonquantitative systematic review; (3) lower quality RCT; (4) clinical cohort study; (5) case-controlled study; (6) historical uncontrolled study; (7) epidemiologic study.
Here are some examples of the way evidence ratings should appear in the text:
“To improve morbidity and mortality, most patients in congestive heart failure should be treated with an angiotensin-converting enzyme inhibitor. [Evidence level A, RCT]”
“The USPSTF recommends that clinicians routinely screen asymptomatic pregnant women 25 years and younger for chlamydial infection. [Evidence level B, non-randomized clinical trial]”
“The American Diabetes Association recommends screening for diabetes every three years in all patients at high risk of the disease, including all adults 45 years and older. [Evidence level C, expert opinion]”
When scientifically strong evidence does not exist to support a given clinical recommendation, you can point this out in the following way:
“Physical therapy is traditionally prescribed for the treatment of adhesive capsulitis (frozen shoulder), although there are no randomized outcomes studies of this approach.”
Format of the Review
Introduction.
The introduction should define the topic and purpose of the review and describe its relevance to family practice. The traditional way of doing this is to discuss the epidemiology of the condition, stating how many people have it at one point in time (prevalence) or what percentage of the population is expected to develop it over a given period of time (incidence). A more engaging way of doing this is to indicate how often a typical family physician is likely to encounter this problem during a week, month, year, or career. Emphasize the key CME objectives of the review and summarize them in a separate table entitled “CME Objectives.”
The methods section should briefly indicate how the literature search was conducted and what major sources of evidence were used. Ideally, indicate what predetermined criteria were used to include or exclude studies (e.g., studies had to be independently rated as being high quality by an established evaluation process, such as the Cochrane Collaboration). Be comprehensive in trying to identify all major relevant research. Critically evaluate the quality of research reviewed. Avoid selective referencing of only information that supports your conclusions. If there is controversy on a topic, address the full scope of the controversy.
The discussion can then follow the typical format of a clinical review article. It should touch on one or more of the following subtopics: etiology, pathophysiology, clinical presentation (signs and symptoms), diagnostic evaluation (history, physical examination, laboratory evaluation, and diagnostic imaging), differential diagnosis, treatment (goals, medical/surgical therapy, laboratory testing, patient education, and follow-up), prognosis, prevention, and future directions.
The review will be comprehensive and balanced if it acknowledges controversies, unresolved questions, recent developments, other viewpoints, and any apparent conflicts of interest or instances of bias that might affect the strength of the evidence presented. Emphasize an evidence-supported approach or, where little evidence exists, a consensus viewpoint. In the absence of a consensus viewpoint, you may describe generally accepted practices or discuss one or more reasoned approaches, but acknowledge that solid support for these recommendations is lacking.
In some cases, cost-effectiveness analyses may be important in deciding how to implement health care services, especially preventive services. 10 When relevant, mention high-quality cost-effectiveness analyses to help clarify the costs and health benefits associated with alternative interventions to achieve a given health outcome. Highlight key points about diagnosis and treatment in the discussion and include a summary table of the key take-home points. These points are not necessarily the same as the key recommendations, whose level of evidence is rated, although some of them will be.
Use tables, figures, and illustrations to highlight key points, and present a step-wise, algorithmic approach to diagnosis or treatment when possible.
Rate the evidence for key statements, especially treatment recommendations. We expect that most articles will have at most two to four key statements; some will have none. Rate only those statements that have corresponding references and base the rating on the quality and level of evidence presented in the supporting citations. Use primary sources (original research, RCTs, meta-analyses, and systematic reviews) as the basis for determining the level of evidence. In other words, the supporting citation should be a primary research source of the information, not a secondary source (such as a nonsystematic review article or a textbook) that simply cites the original source. Systematic reviews that analyze multiple RCTs are good sources for determining ratings of evidence.
The references should include the most current and important sources of support for key statements (i.e., studies referred to, new information, controversial material, specific quantitative data, and information that would not usually be found in most general reference textbooks). Generally, these references will be key evidence-based recommendations, meta-analyses, or landmark articles. Although some journals publish exhaustive lists of reference citations, AFP prefers to include a succinct list of key references. (We will make more extensive reference lists available on our Web site or provide links to your personal reference list.)
You may use the following checklist to ensure the completeness of your evidence-based review article; use the source list of reviews to identify important sources of evidence-based medicine materials.
Checklist for an Evidence-Based Clinical Review Article
The topic is common in family practice, especially topics in which there is new, important information about diagnosis or treatment.
The introduction defines the topic and the purpose of the review, and describes its relevance to family practice.
A table of CME objectives for the review is included.
The review states how you did your literature search and indicates what sources you checked to ensure a comprehensive assessment of relevant studies (e.g., MEDLINE, the Cochrane Collaboration Database, the Center for Research Support, TRIP Database).
Several sources of evidence-based reviews on the topic are evaluated ( Table 1 ) .
Where possible, POEM (dealing with changes in morbidity, mortality, or quality of life) rather than DOE (dealing with mechanistic explanations or surrogate end points, such as changes in laboratory tests) is used to support key clinical recommendations ( Table 2 ) .
Studies of patients likely to be representative of those in primary care practices, rather than subspecialty referral centers, are emphasized.
Studies that are not only statistically significant but also clinically significant are emphasized; e.g., interventions with meaningful changes in absolute risk reduction and low numbers needed to treat. (See http://www.cebm.net/index.aspx?o=1116 .) 11
The level of evidence for key clinical recommendations is labeled using the following rating scale: level A (RCT/meta-analysis), level B (other evidence), and level C (consensus/expert opinion).
Acknowledge controversies, recent developments, other viewpoints, and any apparent conflicts of interest or instances of bias that might affect the strength of the evidence presented.
Highlight key points about diagnosis and treatment in the discussion and include a summary table of key take-home points.
Use tables, figures, and illustrations to highlight key points and present a step-wise, algorithmic approach to diagnosis or treatment when possible.
Emphasize evidence-based guidelines and primary research studies, rather than other review articles, unless they are systematic reviews.
The essential elements of this checklist are summarized in Table 3 .
| Choose a common, important topic in family practice. |
| Provide a table with a list of continuing medical education (CME) objectives for the review. |
| State how the literature search and reference selection were done. |
| Use several sources of evidence-based reviews on the topic. |
| Rate the level of evidence for key recommendations in the text. |
| Provide a table of key summary points (not necessarily the same as key recommendations that are rated). |
Siwek J. Reading and evaluating clinical review articles. Am Fam Physician. 1997;55:2064-2069.
Shaughnessy AF, Slawson DC. Getting the most from review articles: a guide for readers and writers. Am Fam Physician. 1997;55:2155-60.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-17.
Flynn CA, D'Amico F, Smith G. Should we patch corneal abrasions? A meta-analysis. J Fam Pract. 1998;47:264-70.
Slawson DC, Shaughnessy AF, Bennett JH. Becoming a medical information master: feeling good about not knowing everything. J Fam Pract. 1994;38:505-13.
Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-99.
Slawson DC, Shaughnessy AF. Becoming an information master: using POEMs to change practice with confidence. Patient-oriented evidence that matters. J Fam Pract. 2000;49:63-7.
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Methods Work Group, Third U.S. Preventive Services Task Force. Current methods of the U.S. Preventive Services Task Force. A review of the process. Am J Prev Med. 2001;20(3 suppl):21-35.
CATbank topics: levels of evidence and grades of recommendations. Retrieved November 2001, from: http://www.cebm.net/ .
Saha S, Hoerger TJ, Pignone MP, Teutsch SM, Helfand M, Mandelblatt JS. for the Cost Work Group of the Third U.S. Preventive Services Task Force. The art and science of incorporating cost effectiveness into evidence-based recommendations for clinical preventive services. Am J Prev Med. 2001;20(3 suppl):36-43.
Evidence-based medicine glossary. Retrieved November 2001, from: http://www.cebm.net/index.aspx?o=1116 .
Continue Reading
More in afp, more in pubmed.
Copyright © 2002 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- STFM Journals
- Family Medicine
- Annals of Family Medicine

- Recently Published
- Collections
ORIGINAL ARTICLES
Evidence-based medicine culture, curriculum, and program outcomes: a cera study, kate rowland, md, ms | john w. epling, md, msed | rick guthmann, md, mph | joel j. heidelbaugh, md | martha johnson, md, ms | georgia luckey, phd, ms | robert martin, md.
Published: 8/23/2024 | DOI: 10.22454/FamMed.2024.895739
Background: Limited faculty development is a barrier to advancing evidence-based medicine (EBM) education. This study sought to describe program director perception of EBM culture in family medicine residency training and to assess the association among structured faculty roles, EBM curricula, and specific resident outcomes including publications in EBM.
Methods: Members of the Society of Teachers of Family Medicine EBM collaborative drafted survey questions based on a literature review. The questions were electronically distributed in May 2023 to all US family medicine residency program directors who had not previously opted out by the Council of Academic Family Medicine Educational Research Alliance within its study of family medicine program directors. We analyzed results using descriptive and comparative statistics.
Results: The overall response rate was 44.7% (309/691). We found that 260/281 (92%) of program directors reported an EBM curriculum of some kind, and 253/281 (90%) of program directors agreed/strongly agreed that EBM was accepted by residents. Of the respondents, 72/281 (25.6%) reported that no specific faculty member was responsible for their EBM curriculum. Most program directors reported that less than 50% of residents will leave their programs with the ability to detect an error in original research (23.8%; 67/281), detect an important omission in an UpToDate article (16%; 45/281), or author a narrative review for American Family Physician (10%; 28/281).
Conclusions: Program directors reported strong acceptance of EBM among residents and a high prevalence of a formal curriculum. However, many lacked a specific faculty lead, and few reported that residents had strong EBM skills. This study identified gaps in residency training to support future EBM-skilled family physicians as well as concerns about pathways for the development of future EBM faculty.
INTRODUCTION
Evidence-based medicine (EBM) is the application of the best available research to the care of an individual patient. Since the 1990s, EBM has been conceived and taught as critical appraisal of original research for the purpose of answering a clinical point-of-care question. 1 The concept has evolved and expanded to include lifelong learning, information management, and evidence synthesis. EBM is an integral part of family medicine ideals: shared decision-making and value-based care. Shared decision-making requires sufficient knowledge of high-quality evidence as well as skill to counsel a patient on the risks and benefits for their individual situation. Value-based care relies on evidence to support its emphasis on cost-effectiveness. Despite these aspirational ideals, evidence has suggested that family physicians experience substantial barriers to teaching, learning, and exercising evidence-based practices. 2 - 5
The best methods for teaching EBM are not clear. Previous studies were limited by changing resources, evolving technology, and methodologic constructs over the past 20 years. 6 - 8 Traditionally, many family medicine residency programs have relied on curricula emphasizing only one facet of EBM, such as point-of-care questions and answers or critical appraisal of original research. 9 Longitudinal curricula with varying degrees of clinical integration have been studied, but whether they are more effective at producing family medicine residents with confidence and skills at incorporating EBM into practice is unclear. 9 - 12 Few of the existing studies have included behavioral outcomes (ie, outcomes other than knowledge or skill). In addition to didactic curricula, practical experience with advanced EBM skills such as research, authoring, or previous EBM training may be associated with increased EBM skills. 13 - 15 Although critical, this experience, particularly research interest, is negatively correlated with an interest in family medicine. 16
Research conducted in 2015 demonstrated that family medicine residencies value evidence-based practice, and family medicine program directors reported a strong culture of EBM. 17 However, a lack of opportunities for faculty development has been shown to be a limitation to advancing EBM education. 18 Faculty skill, time for EBM teaching, and, in some cases, a perceived tension between patient-centered care and evidence-based care, are other identified barriers. 17 , 19 Literature also has shown a lack of change in self-reported EBM skills during training among family medicine residents. 14
This study sought to describe family medicine program director perceptions of EBM and to report on EBM practices in family medicine residency training. We further sought to assess the association among structured faculty roles and EBM curricula to specific resident outcomes such as resident ability to identify errors in research and resident preparation to author review articles.
Participants
Between April 18 and May 12, 2023, family medicine program directors of US programs accredited by the Accreditation Council for Graduate Medical Education (ACGME) were invited to participate in the Council of Academic Family Medicine Educational Research Alliance (CERA) study. 20 The survey was sent electronically to all family medicine program directors who had not previously opted out. Escalating reminders were sent for a total of five invitations over 4 weeks.
Survey Development
The survey questions were developed by members of the research team based on hypotheses, expert opinion, and literature review. They were reviewed by all members of the research team and revised iteratively to achieve the 10 questions allowed on the omnibus survey. Items assessed family medicine program directors’ perceptions of EBM curriculum, EBM faculty experience and expertise, EBM program culture, and resident EBM outcomes. Program directors reported on faculty expertise (novice to national recognition) and faculty years of experience with EBM.
The CERA steering committee evaluated all questions for reliability and validity based on evidence presented. A sample of family medicine educators who were not part of the survey pretested the questions.
We analyzed survey data with Statistical Analysis Software version 9.4 (SAS Institute) using descriptive statistics and independent ꭓ 2 tests for associations. Missing responses were excluded from analyses, and valid percentages were reported. Response options were collapsed for independent ꭓ 2 analyses due to the small numbers of responses to several options.
Ethics Approval
The project was approved by the American Academy of Family Physicians Institutional Review Board in April 2023.
The overall response rate for the survey was 44.72% (309/691; Table 1).
Table 1: Demographic Information*
|
|
|
| University-based | 48 (16.2) |
| Community-based, university-affiliated | 171 (57.6) |
| Community-based, nonaffiliated | 70 (23.6) |
| Military | 1 (0.3) |
| Other | 7 (2.4) |
|
|
|
| New England (NH, MA, ME, VT, RI, or CT) | 8 (2.6) |
| Middle Atlantic (NY, PA, or NJ) | 46 (14.9) |
| South Atlantic (PR, FL, GA, SC, NC, VA, DC, WV, DE, or MD) | 51 (16.5) |
| East South Central (KY, TN, MS, or AL) | 15 (4.9) |
| East North Central (WI, MI, OH, IN, or IL) | 48 (15.5) |
| West South Central (OK, AR, LA, or TX) | 36 (11.7) |
| West North Central (ND, MN, SD, IA, NE, KS, or MO) | 31 (10) |
| Mountain (MT, ID, WY, NV, UT, AZ, CO, or NM) | 27 (8.7) |
| Pacific (WA, OR, CA, AK, or HI) | 47 (15.2) |
|
|
|
| Less than 30,000 | 33 (11.1) |
| 30,000 to 74,999 | 44 (14.8) |
| 75,000 to 149,000 | 60 (20.1) |
| 150,000 to 499,999 | 74 (24.8) |
| 500,000 to 1 million | 36 (12.1) |
| More than 1 million | 51 (17.1) |
|
|
|
| <19 | 120 (40.4) |
| 19–31 | 133 (44.8) |
| >31 | 44 (14.8) |
|
|
|
| MD | 238 (79.9) |
| DO | 60 (20.1) |
|
|
|
|
|
|
|
|
|
| Female/woman | 162 (55.1) |
| Male/man | 129 (43.9) |
| Genderqueer/gender nonconforming | 0 |
| Nonbinary | 0 |
| Choose not to disclose | 3 (1.0) |
| Self-described | 0 |
*Categorical variable data are presented as counts and percentages (n [%]). Continuous data are presented as median; mean (standard deviation).
Table 1, Continued
|
|
|
| American Indian/Alaska Native/Indigenous | 0 |
| Asian | 28 (9.4) |
| Black/African American | 15 (5.1) |
| Hispanic/Latino/of Spanish Origin | 20 (6.7) |
| Middle Eastern/North African | 3 (1.0) |
| Native Hawaiian/Other Pacific Islander | 0 |
| White | 212 (71.4) |
| Checked multiple race/ethnicities | 10 (3.4) |
| Choose not to disclose | 9 (3.0) |
|
|
|
| No | 235 (80.2) |
| Yes | 58 (19.8) |
Curriculum Outcomes
A total of 42.0% (118/281) of program directors reported that EBM was formalized into both didactics and clinical experiences. And 37.0% (104/281) reported that EBM was formalized into didactics only: 24.2% into didactics beyond journal club and 12.8% into journal club only (Table 2).
Table 2: Survey Responses
|
|
|
| ) |
|
| No specific person | 72 (25.6) |
| Limited experience with EBM topics | 18 (6.4) |
| Mostly comfortable with EBM topics | 97 (34.5) |
| Regional/local recognition or presentations | 54 (19.2) |
| National recognition, journal editor, publishes/presents on EBM topics | 40 (14.2) |
|
|
|
| No specific person | 74 (26.4) |
| 0–4 years in practice | 31 (11.1) |
| 5–10 years in practice | 64 (22.9) |
| 11–15 years in practice | 45 (16.1) |
| 16+ years in practice | 66 (23.6) |
|
|
|
| We don’t have an EBM faculty member. | 75 (26.7) |
| We could replace them with another current faculty member. | 88 (31.3) |
| It would take <3 months to identify a replacement faculty member. | 19 (6.8) |
| It would take 3 to <6 months to identify a replacement faculty member. | 22 (7.8) |
| It would take 6 to <12 months to identify a replacement faculty member. | 33 (11.7) |
| It would take ≥12 months to identify a replacement faculty member. | 44 (15.7) |
|
|
|
| We do not have a formal EBM curriculum. | 21 (7.5) |
| Incorporated into didactic experiences (journal club only) | 36 (12.8) |
| Incorporated into didactic experiences (beyond journal club) | 68 (24.2) |
| Informally incorporated into clinical experiences | 22 (7.8) |
| Formalized into clinical experiences | 7 (2.5) |
| Formalized into both didactics and clinical experiences | 118 (42.0) |
| Another model | 9 (3.2) |
|
|
|
| Strongly disagree | 6 (2.1) |
| Disagree | 5 (1.8) |
| Neutral | 16 (5.7) |
| Agree | 116 (41.4) |
| Strongly agree | 137 (48.9) |
Table 2, continued
|
|
|
|
|
|
| Strongly disagree | 6 (2.1) |
| Disagree | 3 (1.1) |
| Neutral | 17 (6.0) |
| Agree | 98 (34.9) |
| Strongly agree | 157 (55.9) |
|
|
|
| Strongly disagree | 111 (39.5) |
| Disagree | 123 (43.8) |
| Neutral | 24 (8.5) |
| Agree | 17 (6.0) |
| Strongly agree | 6 (2.1) |
|
|
|
| None | 8 (2.8) |
| 1% | 12 (4.3) |
| >1% to <25% | 94 (33.5) |
| 25% to <50% | 100 (35.6) |
| ≥50% | 67 (23.8) |
|
|
|
| None | 17 (6.1) |
| 1% | 9 (3.2) |
| >1% to <25% | 117 (41.9) |
| 25% to <50% | 91 (32.6) |
| ≥50% | 45 (16.1) |
|
|
|
| None | 32 (11.4) |
| 1% | 29 (10.3) |
| >1% to <25% | 134 (47.7) |
| 25% to <50% | 58 (20.6) |
| ≥50% | 28 (10.0) |
EBM Culture Outcomes
More than 90% of program directors agree/strongly agree that both faculty (255/281; 90.7%) and residents (253/280; 90.3%) accept the process and outcome of an evidence search for answers to clinical questions. Approximately 83% (234/281) disagree/strongly disagree that clinicians find reasons to doubt or reject evidence, or avoid incorporating it into practice.
Resident Competence Outcomes
Of the 309 program directors, 264 (84.9%) reported that fewer than 50% of the residents in their program would be able to identify a major error or omission in an UpToDate article. And 143/309 (46.3%) reported that fewer than 25% would be able to do so.
We found that 214/309 (69.3%) of program directors reported that fewer than 50% of their residents would be able to identify a significant error in an original research study; 114/309 (36.9%) reported that fewer than 25% would be able to do so.
Twenty-one percent (61/309) of program directors reported that 0–1 of their current residents will graduate with the skills to be the lead author on a narrative review article. Ten percent (28/309) of program directors reported that at least 50% of their residents will be able to do so.
Faculty Outcomes
A total of 25.6% (72/309) of program directors reported that no identifiable person is leading an EBM curriculum at their program. Among the program directors, 23.6% reported that their EBM faculty has been in practice at least 16 years. Faculty experience greater than 16 years was associated with formalized EBM curriculum in both didactics and clinical experiences, or clinical experiences only ( P <.001; Table 3).
Table 3: Faculty Years in Practice × EBM Curriculum Format
|
|
| ||||
|
|
|
|
|
| |
|
|
|
|
|
|
|
| We do not have a formal EBM curriculum. | 16 (21.6) | 2 (6.5) | 2 (3.1) | 1 (2.2) | 0 |
| Incorporated into didactic experiences (journal club only) | 8 (10.8) | 10 (32.3) | 5 (7.8) | 4 (8.9) | 8 (12.1) |
| Incorporated into didactic experiences (beyond journal club) | 15 (20.3) | 7 (22.6) | 17 (26.6) | 15 (33.3) | 14 (21.2) |
| Informally incorporated into clinical experiences (eg, residents are encouraged to look things up, but formal presentations are not scheduled) | 8 (10.8) | 2 (6.5) | 6 (9.4) | 3 (6.7) | 3 (4.5) |
| Formalized into clinical experiences (eg, morning report includes a literature review and presentation of primary research) | 2 (2.7) | 0 | 2 (3.1) | 2 (4.4) | 1 (1.5) |
| Formalized into both didactics and clinical experiences | 24 (32.4) | 10 (32.3) | 28 (43.8) | 19 (42.2) | 37 (56.1) |
| Another model | 1 (1.4) | 0 | 4 (6.3) | 1 (2.2) | 3 (4.5) |
* P <.001
Note: Using a ꭓ 2 test of independence, a significant association between EBM faculty years in practice and EBM curriculum was found ( P <.0001).
Abbreviation: EBM, evidence-based medicine
We found a significant association between EBM faculty years in practice and expertise level of the faculty member ( P =.0008). Program directors reported that 32% of EBM faculty members with 16 or more years of experience have national recognition compared to 18% of EBM faculty members with 11 to 15 years of experience, 17% with 5 to 10 years, and 11% with 0 to 4 years.
Only 31% of program directors reported that they could replace current EBM faculty from within their own faculty; 15.7% reported that it would take 12 months or more to do so.
The absence of a designated EBM faculty member was found to correlate with the absence of a formal EBM curriculum ( P <.001). Of program directors that responded they had no specific EBM faculty lead, 22% (16/72) reported that they did not have a formal EBM curriculum, compared with 5/209 (2.4%) of programs with an EBM faculty lead.
Associations Between Faculty and Curriculum Factors and Resident Outcomes
Faculty years in practice were not associated with resident competency outcomes; more experienced faculty were not associated with improved residency outcomes (Table 4). The type of EBM curriculum (eg, formal or informal, didactic only or clinically integrated) was not associated with identifying omissions in UpToDate or authoring narrative reviews but was associated with identifying errors in original research ( P =.0228; Table 5).
Table 4: Faculty Years in Practice × Resident Outcomes
|
|
| ||||
|
|
|
|
|
| |
|
|
|
|
|
|
|
| None | 3 (4.1) | 0 | 2 (3.1) | 1 (2.2) | 2 (3.0) |
| 1% | 1 (1.4) | 2 (6.5) | 3 (4.7) | 3 (6.7) | 3 (4.5) |
| >1% to <25% | 31 (41.9) | 15 (48.4) | 16 (25) | 9 (20.0) | 22 (33.3) |
| 25% to <50% | 21 (28.4) | 9 (29.0) | 30 (46.9) | 14 (31.1) | 26 (39.4) |
| ≥50% | 18 (24.3) | 5 (16.1) | 13 (20.3) | 18 (40) | 13 (19.7) |
|
|
|
|
|
|
|
| None | 6 (8.1) | 3 (9.7) | 3 (4.7) | 1 (2.2) | 4 (6.1) |
| 1% | 1 (1.4) | 2 (6.5) | 3 (4.7) | 2 (4.4) | 1 (1.5) |
| >1% to <25% | 32 (43.2) | 15 (48.4) | 28 (43.8) | 16 (35.6) | 25 (37.9) |
| 25% to <50% | 21 (28.4) | 9 (29.0) | 20 (31.3) | 18 (40) | 23 (34.8) |
| ≥50% | 13 (17.6) | 2 (6.5) | 10 (15.6) | 7 (15.6) | 13 (19.7) |
|
|
|
|
|
|
|
| None | 6 (8.1) | 4 (12.9) | 9 (14.1) | 7 (15.6) | 6 (9.1) |
| 1% | 11 (14.9) | 4 (12.9) | 5 (7.8) | 3 (6.7) | 6 (9.1) |
| >1% to <25% | 36 (48.6) | 15 (48.4) | 31 (48.4) | 22 (48.9) | 29 (43.9) |
| 25% to <50% | 17 (23) | 5 (16.1) | 12 (18.8) | 7 (15.6) | 17 (25.8) |
| ≥50% | 4 (5.4) | 3 (9.7) | 7 (10.9) | 6 (13.3) | 8 (12.1) |
Note: No significant association exists between faculty years in practice and any of the resident outcomes: identifying a major omission in UpToDate, authoring a review article, or identifying a significant error in an original research article.
Table 5: EBM Curriculum Format × Resident Outcomes
|
|
| ||||||
|
|
|
|
|
|
|
|
|
|
| |||||||
|
|
|
|
|
|
|
|
|
| None | 2 (10) | 3 (8) | 1 (1) | 0 | 1 (14) | 1 (1) | 0 |
| 1% | 2 (10) | 3 (8) | 2 (3) | 0 | 0 | 4 (3) | 1 (11) |
| >1% to <25% | 6 (29) | 14 (39) | 22 (32) | 9 (41) | 3 (43) | 35 (30) | 5 (56) |
| 25% to <50% | 8 (38) | 11 (31) | 31 (46) | 7 (32) | 1 (14) | 39 (33) | 3 (33) |
| ≥50% | 3 (14) | 5 (14) | 12 (18) | 6 (27) | 2 (29) | 39 (33) | 0 |
|
|
|
|
|
|
|
|
|
| None | 3 (14) | 4 (11) | 2 (3) | 1 (5) | 1 (14) | 5 (4) | 1 (11) |
| 1% | 0 | 2 (6) | 4 (6) | 0 | 0 | 2 (2) | 1 (11) |
| >1% to <25% | 6 (29) | 19 (53) | 31 (46) | 9 (41) | 3 (43) | 45 (38) | 4 (44) |
| 25% to <50% | 7 (33) | 8 (22) | 21 (31) | 9 (41) | 1 (14) | 43 (36) | 2 (22) |
| ≥50% | 5 (24) | 3 (8) | 10 (15) | 3 (14) | 2 (29) | 21 (18) | 1 (11) |
|
|
|
|
|
|
|
|
|
| None | 2 (10) | 7 (19) | 8 (12) | 4 (18) | 0 | 9 (8) | 2 (22) |
| 1% | 2 (10) | 9 (25) | 7 (10) | 0 | 1 (14) | 10 (9) | 0 |
| >1% to <25% | 12 (57) | 14 (39) | 30 (44) | 14 (64) | 3 (43) | 55 (47) | 6 (67) |
| 25% to <50% | 4 (19) | 3 (8) | 18 (27) | 2 (9) | 0 | 30 (25) | 1 (11) |
| ≥50% | 1 (5) | 3 (8) | 5 (7) | 2 (9) | 3 (43) | 14 (12) | 0 |
* P <.05
Note: No significant correlation was found between EBM curriculum format and identifying an omission in UpToDate or being able to author an American Family Physician article, but we found a significant association between EBM curriculum and identifying an error in original research ( P <.05).
Abbreviations: EBM, evidence-based medicine; NFC, no formal curriculum; JCO, journal club only; BJC, beyond journal club
More than 90% of program directors strongly agreed or agreed that EBM is accepted by residents and faculty, and that their programs have an EBM curriculum. However, the reported acceptance of EBM and integration of EBM curricula are contrasted with program directors’ reporting of resident outcomes. Having program directors define the nature of their curriculum was not within the scope of our survey. Program directors reported that far fewer than one-half of current residents can identify a significant error in original research or a major omission in a resource such as UpToDate. These factors were included in the survey because they are consistent with expectations from the ACGME requirements, which call for residents to be able to “challenge the evidence” used to make decisions and to “understand the benefits and limitations of the medical literature.” 21 Family physicians regularly rely on point-of-care and nonevidence-based tools such as UpToDate; yet, without the skills to identify important omissions or errors, we are dependent on the tools’ own assessment of trustworthiness and accuracy. This may negatively impact patient care.
This study showed that we are not graduating residents who are capable of identifying serious errors in research studies, can appropriately critique secondary sources, or can contribute to authoring evidence-based literature. Few program directors reported that their residents will graduate prepared to author an EBM-based narrative review article. Though family physicians rely on high-quality evidence-based reviews, residencies are not consistently training graduates to author, edit, and publish such works. A major aim in training family medicine residents is to have them learn from original research, ideally from EBM-trained experts through didactics, journal clubs, and patient encounters. This deficiency presents a missed opportunity for family physicians to become leaders and influencers among their peers, and it also raises concern about the development of current and future EBM faculty.
ACGME requires family medicine residents to demonstrate the ability to appraise and assimilate scientific evidence and to use that evidence to develop a patient care plan. A regular forum for discussing and analyzing evidence relevant to practice is also a core requirement. 21 Our study determined that about 25% of program directors reported having no specific faculty member responsible for their EBM curriculum. Programs without a faculty EBM lead were less likely to report having a formal EBM curriculum. Prior studies of EBM curricula have reported that limited time to teach EBM skills and difficulty recruiting EBM-skilled teachers are the largest barriers to implementing an EBM curriculum. 9 This study also found that program directors with more experienced EBM faculty members are more likely to report that their EBM curriculum is integrated into clinical practice. Taken together, these findings suggest that having designated faculty members and opportunities for ongoing faculty development are more likely to lead to integrated EBM curricula consistent with ACGME requirements.
Years of experience of the EBM lead faculty was not associated with any of the resident outcomes in this study. This finding suggests that more senior faculty are not more likely to produce better outcomes. Junior faculty can lead EBM curricula as successfully as senior faculty, although outcomes across the spectrum of faculty experience and expertise are lacking. As noted, the pathways for future designated EBM faculty development are of concern and are not well-defined. Only 11% of program directors reported having a faculty member with 0 to 4 years of experience responsible for their EBM curriculum. This finding, coupled with more than 26% of programs without a designated faculty member, suggests that significantly more resources and support for junior faculty and faculty development are needed. Clearly defined faculty development competencies would align family medicine EBM faculty around key areas of skill development. Similarly, the need to improve resident ability at critical appraisal of primary and secondary literature sources and evidence synthesis should drive the creation of resident-level competencies in EBM.
Our study demonstrated that while EBM skills are valued as necessary and integral to the practice of family medicine, most residency training programs lack sufficient faculty expertise and curricula to teach these skills in line with ACGME standards. This deficit ultimately will lead to continued reliance on nonevidence-based resources in clinical practice. Integration of robust EBM training is imperative to ensure that the upcoming generation of junior faculty will possess skills in EBM teaching, because our study found that nearly one-third of EBM-competent faculty have more than 16 years of faculty experience.
Limitations
This was a self-report survey of program directors based on their perceptions of current faculty and expertise, and results may not correlate with the opinions of the EBM faculty or the residents themselves. Results may be subject to self-reporting biases, including social desirability bias and recall bias. The response rate of the survey was 44.7%, and we do not have information on nonresponders. Details regarding an EBM curriculum were not specifically defined, thus we assume moderate variability and heterogeneity across programs.
As a cross-sectional design, this study provides insight into a single point in time. Some analyses were affected by the need to group some responses due to the small number of responses to several options. In some cases, even after grouping, the validity of these analyses was still affected, and this should be taken into consideration when interpreting the findings.
Residency programs widely vary in cohort size and number of faculty, and some programs may have more than one identified EBM faculty member, which could include the program director. Our study was not designed to capture those data.
CONCLUSIONS
Family medicine residency program directors reported strong resident and faculty acceptance of EBM. However, they reported that few residents are graduating with EBM skills adequate for clinical practice. Many program directors reported not having an identified faculty member responsible for the EBM curriculum. Further study about effective teaching of EBM for residents and curriculum best practices is needed to foster integration of EBM resources and clinical practice. Development of continuing medical education and faculty development will help ensure a pipeline of effective future and current EBM faculty and will help meet ACGME core requirements for resident outcomes.
Presentations
This study was presented at the 2024 Annual Meeting of the Society of Teachers of Family Medicine in Los Angeles, CA.
- Zimerman AL. Evidence-based medicine: a short history of a modern medical movement. Virtual Mentor . 2013;15(1):71-76.
- Swennen MHJ, van der Heijden, GJMG, Boeije HR, et al. Doctors’ perceptions and use of evidence-based medicine: a systematic review and thematic synthesis of qualitative studies. Acad Med. 2013;88(9):1,384-1,396.
- van Dijk N, Hooft L, Wieringa-de Waard M. What are the barriers to residents’ practicing evidence-based medicine? a systematic review. Acad Med. 2010;85(7):1,163-1,170.
- Lafuente-Lafuente C, Leitao C, Kilani I, et al. Knowledge and use of evidence-based medicine in daily practice by health professionals: a cross-sectional survey. BMJ Open . 2019;9(3):e025224. doi:10.1136/bmjopen-2018-025224
- Hisham R, Ng CJ, Liew SM, Hamzah N, Ho GJ. Why is there variation in the practice of evidence-based medicine in primary care? a qualitative study. BMJ Open . 2016;6(3):e010565. doi:10.1136/bmjopen-2015-010565
- Ahmadi N, McKenzie ME, Maclean A, Brown CJ, Mastracci T, McLeod RS. Teaching evidence based medicine to surgery residents—is journal club the best format? a systematic review of the literature. J Surg Educ . 2012;69(1):91-100. doi:10.1016/j.jsurg.2011.07.004
- Bednarczyk J, Pauls M, Fridfinnson J, Weldon E. Characteristics of evidence-based medicine training in Royal College of Physicians and Surgeons of Canada emergency medicine residencies—a national survey of program directors. BMC Med Educ . 2014;14(1):57. doi:10.1186/1472-6920-14-57
- Carpenter CR, Kane BG, Carter M, Lucas R, Wilbur LG, Graffeo CS. Incorporating evidence-based medicine into resident education: a CORD survey of faculty and resident expectations. Acad Emerg Med . 2010;17(Suppl 2):S54-S61. doi:10.1111/j.1553-2712.2010.00889.x
- Halalau A, Holmes B, Rogers-Snyr A, et al. Evidence-based medicine curricula and barriers for physicians in training: a scoping review. Int J Med Educ . 2021;12:101-124. doi:10.5116/ijme.6097.ccc0
- Song C, Porcello L, Hernandez T, Levandowski BA. Description and evaluation of an evidence-based residency curriculum using the evidence-based medicine environment survey. Fam Med . 2022;54(4):298-303. doi:10.22454/FamMed.2022.652106
- Ilic D, Maloney S. Methods of teaching medical trainees evidence-based medicine: a systematic review. Med Educ . 2014;48(2):124-135. doi:10.1111/medu.12288
- Coomarasamy A, Khan KS. What is the evidence that postgraduate teaching in evidence based medicine changes anything? a systematic review. BMJ . 2004;329(7473):1,017-1,019. doi:10.1136/bmj.329.7473.1017
- Aneese AM, Nasr JA, Halalau A. A prospective mixed-methods study evaluating the integration of an evidence based medicine curriculum into an internal medicine residency program. Adv Med Educ Pract . 2019;10:533-546. doi:10.2147/AMEP.S203334
- Paulsen J, Al Achkar M. Factors associated with practicing evidence-based medicine: a study of family medicine residents. Adv Med Educ Pract . 2018;9:287-293. doi:10.2147/AMEP.S157792
- Zee M, de Boer M, Jaarsma ADC. Acquiring evidence-based medicine and research skills in the undergraduate medical curriculum: three different didactical formats compared. Perspect Med Educ . 2014;3(5):357-370. doi:10.1007/S40037-014-0143-Y
- Senf JH, Campos-Outcalt D, Kutob R. Family medicine specialty choice and interest in research. Fam Med . 2005;37(4):265-270. https://www.stfm.org/familymedicine/vol37issue4/Senf265
- Epling JW, Heidelbaugh JJ, Woolever D, et al. Examining an evidence-based medicine culture in residency education. Fam Med . 2018;50(10):751-755. doi:10.22454/FamMed.2018.576501
- Lehane E, Leahy-Warren P, O’Riordan C, et al. Evidence-based practice education for healthcare professions: an expert view. BMJ Evid Based Med . 2019;24(3):103-108. doi:10.1136/bmjebm-2018-111019
- Zwolsman S, te Pas E, Hooft L, Wieringa-de Waard M, van Dijk N. Barriers to GPs’ use of evidence-based medicine: a systematic review. Br J Gen Pract . 2012;62(600):e511-e521.
- Seehusen DA, Mainous AG, Chessman AW. Creating a centralized infrastructure to facilitate medical education research. Ann Fam Med . 2018;16(3):257-260. doi:10.1370/afm.2228
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). ACGME; 2023. Accessed August 19, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
Lead Author
Kate Rowland, MD, MS
Affiliations: Department of Family and Preventive Medicine, Chicago, IL
John W. Epling, MD, MSEd - Department of Family and Community Medicine, Virginia Tech Carilion School of Medicine, Roanoke, VA
Rick Guthmann, MD, MPH - Advocate Illinois Masonic Family Medicine Residency, Chicago, IL
Joel J. Heidelbaugh, MD - Department of Family Medicine, Michigan Medicine, University of Michigan, Ann Arbor, MI
Martha Johnson, MD, MS - Franklin Square Family Medicine Residency Program, MedStar Health, Baltimore, MD
Georgia Luckey, PhD, MS - Department of Family and Community Medicine, School of Medicine, Southern Illinois University, Springfield, IL
Robert Martin, MD - Advocate Illinois Masonic Family Medicine Residency, Chicago, IL
Corresponding Author
Correspondence: Department of Family and Preventive Medicine, 1700 W. Van Buren, Chicago IL 60612, 312-942-9442
Email: [email protected]
There are no comments for this article.
Downloads & info, related content.
Rowland K, Epling JW, Guthmann R, et al. Evidence-Based Medicine Culture, Curriculum, and Program Outcomes: A CERA Study. [published August 23, 2024]. Fam Med. https://doi.org/10.22454/FamMed.2024.895739.
Citation files in RIS format are importable by EndNote, ProCite, RefWorks, Mendeley, and Reference Manager.
- RIS (EndNote, Reference Manager, ProCite, Mendeley, RefWorks)
- BibTex (JabRef, BibDesk, LaTeX)
Search Results

Contact STFM
2024 © Society of Teachers of Family Medicine. All Rights Reserved.

Evidence-based Medicine: Types of Literature
- Core EBM Databases
- Ask an Answerable Question (PICO)
- Acquire the Evidence
- Appraise the Evidence
- Apply the evidence in your clinical practice.
- Assess your performance
- Study Designs
Types of Literature
- Clinical Guidelines
- How to Find Books
- PubMed Guide This link opens in a new window
- EBM Resources
- Systematic Review Guide This link opens in a new window
- Literature Review Guide This link opens in a new window
Different types of publications have different characteristics.
Primary Literature Primary sources means original studies, based on direct observation, use of statistical records, interviews, or experimental methods, of actual practices or the actual impact of practices or policies. They are authored by researchers, contains original research data, and are usually published in a peer-reviewed journal. Primary literature may also include conference papers, pre-prints, or preliminary reports. Also called empirical research .
Secondary Literature Secondary literature consists of interpretations and evaluations that are derived from or refer to the primary source literature. Examples include review articles (such as meta-analysis and systematic reviews) and reference works. Professionals within each discipline take the primary literature and synthesize, generalize, and integrate new research.
Tertiary Literature Tertiary literature consists of a distillation and collection of primary and secondary sources such as textbooks, encyclopedia articles, and guidebooks or handbooks. The purpose of tertiary literature is to provide an overview of key research findings and an introduction to principles and practices within the discipline.
Adapted from the Information Services Department of the Library of the Health Sciences-Chicago , University of Illinois at Chicago.
|
Original research results in journals, |
Review articles, systematic reviews, meta-analysis, practice guidelines, monographs on a specific subject |
Textbooks, encyclopedias, handbooks, newspapers |
| Sources: NEJM, JAMA | Sources: PubMed, CINAHL, Cochrane Library, Web of Science, Williams Obstetrics, Hurst's The Heart | Sources: Gale Encyclopedia of Genetic Disorders, Oxford Handbook of Internal Medicine |
Types of Scientific Publications
These examples and descriptions of publication types will give you an idea of how to use various works and why you would want to write a particular kind of paper.
- Scholarly article aka empirical article
- Review article
- Conference paper
Scholarly (aka empirical) article -- example
Empirical studies use data derived from observation or experiment. Original research papers (also called primary research articles) that describe empirical studies and their results are published in academic journals. Articles that report empirical research contain different sections which relate to the steps of the scientific method.
Abstract - The abstract provides a very brief summary of the research.
Introduction - The introduction sets the research in a context, which provides a review of related research and develops the hypotheses for the research.
Method - The method section describes how the research was conducted.
Results - The results section describes the outcomes of the study.
Discussion - The discussion section contains the interpretations and implications of the study.
References - A references section lists the articles, books, and other material cited in the report.
Review article -- example
A review article summarizes a particular field of study and places the recent research in context. It provides an overview and is an excellent introduction to a subject area. The references used in a review article are helpful as they lead to more in-depth research.
Many databases have limits or filters to search for review articles. You can also search by keywords like review article, survey, overview, summary, etc.
Conference proceedings, abstracts and reports -- example
Conference proceedings, abstracts and reports are not usually peer-reviewed. A conference article is similar to a scholarly article insofar as it is academic. Conference articles are published much more quickly than scholarly articles. You can find conference papers in many of the same places as scholarly articles.
How Do You Identify Empirical Articles?
To identify an article based on empirical research, look for the following characteristics:
The article is published in a peer-reviewed journal .
The article includes charts, graphs, or statistical analysis .
The article is substantial in size , likely to be more than 5 pages long.
The article contains the following parts (the exact terms may vary): abstract, introduction, method, results, discussion, references .
- << Previous: Study Designs
- Next: Clinical Guidelines >>
- Last Updated: Aug 16, 2024 4:13 PM
- URL: https://research.library.gsu.edu/EBM
- Evidence-Based Medicine
- Finding the Evidence
- eJournals for EBM
Levels of Evidence
- JAMA Users' Guides
- Tutorials (Learning EBM)
- Web Resources
Resources That Rate The Evidence
- ACP Smart Medicine
- Agency for Healthcare Research and Quality
- Clinical Evidence
- Cochrane Library
- Health Services/Technology Assessment Texts (HSTAT)
- PDQ® Cancer Information Summaries from NCI
- Trip Database
Critically Appraised Individual Articles
- Evidence-Based Complementary and Alternative Medicine
- Evidence-Based Dentistry
- Evidence-Based Nursing
- Journal of Evidence-Based Dental Practice
Grades of Recommendation
| | | |
| A | 1a | Systematic review of (homogeneous) randomized controlled trials |
| A | 1b | Individual randomized controlled trials (with narrow confidence intervals) |
| B | 2a | Systematic review of (homogeneous) cohort studies of "exposed" and "unexposed" subjects |
| B | 2b | Individual cohort study / low-quality randomized control studies |
| B | 3a | Systematic review of (homogeneous) case-control studies |
| B | 3b | Individual case-control studies |
| C | 4 | Case series, low-quality cohort or case-control studies |
| D | 5 | Expert opinions based on non-systematic reviews of results or mechanistic studies |
Critically-appraised individual articles and synopses include:
Filtered evidence:
- Level I: Evidence from a systematic review of all relevant randomized controlled trials.
- Level II: Evidence from a meta-analysis of all relevant randomized controlled trials.
- Level III: Evidence from evidence summaries developed from systematic reviews
- Level IV: Evidence from guidelines developed from systematic reviews
- Level V: Evidence from meta-syntheses of a group of descriptive or qualitative studies
- Level VI: Evidence from evidence summaries of individual studies
- Level VII: Evidence from one properly designed randomized controlled trial
Unfiltered evidence:
- Level VIII: Evidence from nonrandomized controlled clinical trials, nonrandomized clinical trials, cohort studies, case series, case reports, and individual qualitative studies.
- Level IX: Evidence from opinion of authorities and/or reports of expert committee
Two things to remember:
1. Studies in which randomization occurs represent a higher level of evidence than those in which subject selection is not random.
2. Controlled studies carry a higher level of evidence than those in which control groups are not used.
Strength of Recommendation Taxonomy (SORT)
- SORT The American Academy of Family Physicians uses the Strength of Recommendation Taxonomy (SORT) to label key recommendations in clinical review articles. In general, only key recommendations are given a Strength-of-Recommendation grade. Grades are assigned on the basis of the quality and consistency of available evidence.
- << Previous: eJournals for EBM
- Next: JAMA Users' Guides >>
- Last Updated: Aug 16, 2024 4:11 PM
- URL: https://guides.library.stonybrook.edu/evidence-based-medicine
- Request a Class
- Hours & Locations
- Ask a Librarian
- Special Collections
- Library Faculty & Staff
Library Administration: 631.632.7100
- Stony Brook Home
- Campus Maps
- Web Accessibility Information
- Accessibility Barrier Report Form

Comments or Suggestions? | Library Webmaster

Except where otherwise noted, this work by SBU Libraries is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License .
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Evidence-Based Medicine: Literature Reviews
When talking to your patients about complementary health approaches, you want to be able to answer the question: Is there any scientific evidence that this complementary product or practice works and is safe? The resources on this page will help inform you about what the science says (limited to the past 5 years).
For more information please contact the NCCIH Clearninghouse .
- Açaí - Randomized Controlled Trials (PubMed®)
- Açaí - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Acupuncture - Randomized Controlled Trials (PubMed®)
- Acupuncture - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Acupuncture for Chronic Pain - Randomized Controlled Trials (PubMed®)
- Acupuncture for Chronic Pain - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Aging - Randomized Controlled Trials (PubMed®)
- Aging - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Aloe Vera - Herb-Drug Interactions (PubMed®)
- Aloe Vera - Randomized Controlled Trials (PubMed®)
- Aloe Vera - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Alzheimer's Disease / Dementia - Randomized Controlled Trials (PubMed®)
- Alzheimer's Disease / Dementia - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Antioxidants - Randomized Controlled Trials (PubMed®)
- Antioxidants - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Anxiety - Randomized Controlled Trials (PubMed®)
- Anxiety - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Anxiety and Complementary Health Approaches - Randomized Controlled Trials (PubMed®)
- Anxiety and Complementary Health Approaches - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Aromatherapy - Randomized Controlled Trials (PubMed®)
- Aromatherapy - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Artemisinin - Randomized Controlled Trials (PubMed®)
- Artemisinin - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Arthritis - Randomized Controlled Trials (PubMed®)
- Arthritis - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Asthma - Randomized Controlled Trials (PubMed®)
- Asthma - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Attention-Deficit Hyperactivity Disorder (ADHD) - Randomized Controlled Trials (PubMed®)
- Attention-Deficit Hyperactivity Disorder (ADHD) - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Autism - Randomized Controlled Trials (PubMed®)
- Autism - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Autism Spectrum Disorder and Complementary Health Approaches—Randomized Controlled Trials (PubMed®)
- Autism Spectrum Disorder and Complementary Health Approaches—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Avermectin - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Ayurvedic Medicine - Randomized Controlled Trials (PubMed®)
- Ayurvedic Medicine - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Benign Prostatic Hyperplasia and Complementary and Integrative Approaches—Randomized Controlled Trials (PubMed®)
- Benign Prostatic Hyperplasia and Complementary and Integrative Approaches—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Benign Prostatic Hyperplasia and Complementary Health Practices - Randomized Controlled Trials (PubMed®)
- Benign Prostatic Hyperplasia and Complementary Health Practices - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Black Cohosh - Randomized Controlled Trials (PubMed®)
- Black Cohosh - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Black Cohosh: Herb-Drug Interactions (PubMed®)
- Bodybuilding - Randomized Controlled Trials (PubMed®)
- Bodybuilding - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Calcium - Randomized Controlled Trials (PubMed®)
- Calcium - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Cancer - Randomized Controlled Trials (PubMed®)
- Cancer - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Cannabis - Randomized Controlled Trials (PubMed®)
- Cannabis - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Chamomile - Randomized Controlled Trials (PubMed®)
- Chamomile - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Chelation for Coronary Heart Disease - Randomized Controlled Trials (PubMed®)
- Chelation for Coronary Heart Disease - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Children’s Use of Complementary Health Approaches—Randomized Controlled Trials (PubMed®)
- Chiropractic - Randomized Controlled Trials (PubMed®)
- Chiropractic - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Cholesterol - Randomized Controlled Trials (PubMed®)
- Cholesterol - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Chondroitin and Glucosamine - Randomized Controlled Trials (PubMed®)
- Chondroitin and Glucosamine - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Coenzyme Q10 - Randomized Controlled Trials (PubMed®)
- Coenzyme Q10 - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Cold and Flu - Randomized Controlled Trials (PubMed®)
- Cold and Flu - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Common Cold and Complementary Health Approaches—Randomized Controlled Trials (PubMed®)
- Common Cold and Complementary Health Approaches—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Complementary Health Approaches for Chronic Pain—Randomized Controlled Trials (PubMed®)
- Complementary Health Approaches for Chronic Pain—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Complementary Health Approaches for Smoking Cessation—Randomized Controlled Trials (PubMed®)
- Complementary Health Approaches for Smoking Cessation—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Cranberry - Randomized Controlled Trials (PubMed®)
- Cranberry - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Creatine - Randomized Controlled Trials (PubMed®)
- Creatine - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Cupping - Randomized Controlled Trials (PubMed®)
- Cupping - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Depression - Randomized Controlled Trials (PubMed®)
- Depression - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Depression and Complementary Health Approaches—Randomized Controlled Trials (PubMed®)
- Dermatitis, Atopic, Acne, Impetigo, Psoriasis, Rosacea - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Detox Diets - Randomized Controlled Trials (PubMed®)
- Detox Diets - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Diabetes - Randomized Controlled Trials (PubMed®)
- Diabetes - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Dietary Supplements and Cognitive Function—Randomized Controlled Trials (PubMed®)
- Dietary Supplements and Cognitive Function—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Dietary Supplements and Safety - Literature on Dietary Supplements and Safety (PubMed®)
- Dietary Supplements for Eye Conditions—Randomized Controlled Trials (PubMed®)
- Dietary Supplements for Eye Conditions—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Dimethyl Sulfoxide (DMSO) and Methylsulfonylmethane (MSM) for Osteoarthritis - Randomized Controlled Trials (PubMed®)
- Dimethyl Sulfoxide (DMSO) and Methylsulfonylmethane (MSM) for Osteoarthritis - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Echinacea - Herb-Drug Interactions (PubMed®)
- Echinacea - Randomized Controlled Trials (PubMed®)
- Echinacea - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Erectile Dysfunction - Randomized Controlled Trials (PubMed®)
- Erectile Dysfunction - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Evening Primrose Oil - Randomized Controlled Trials (PubMed®)
- Evening Primrose Oil - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Eye Conditions - Randomized Controlled Trials (PubMed®)
- Eye Conditions - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Eye Diseases - Randomized Controlled Trials (PubMed®)
- Eye Diseases - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Fenugreek - Herb-Drug Interactions (PubMed®)
- Fenugreek - Randomized Controlled Trials (PubMed®)
- Fenugreek - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Fibromyalgia - Randomized Controlled Trials (PubMed®)
- Fibromyalgia - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Folic Acid and Pregnancy - Randomized Controlled Trials (PubMed®)
- Folic Acid and Pregnancy - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Garlic - Herb-Drug Interactions (PubMed®)
- Garlic - Randomized Controlled Trials (PubMed®)
- Garlic - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Ginkgo - Randomized Controlled Trials (PubMed®)
- Ginkgo - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Ginkgo: Herb-Drug Interactions (PubMed®)
- Ginseng - Herb-Drug Interactions (PubMed®)
- Ginseng - Randomized Controlled Trials (PubMed®)
- Ginseng - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Headache - Randomized Controlled Trials (PubMed®)
- Headache - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Heart Disease - Randomized Controlled Trials (PubMed®)
- Heart Disease - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Hepatitis C - Randomized Controlled Trials (PubMed®)
- Hepatitis C - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Hepatitis C and Dietary Supplements—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Hepatitis C and Silymarin - Randomized Controlled Trials (PubMed®)
- Hepatitis C and Silymarin - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Herb-Drug Interactions - Randomized Controlled Trials (PubMed®)
- Herb-Drug Interactions - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Hypertension - Randomized Controlled Trials (PubMed®)
- Hypertension - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Hypnosis - Randomized Controlled Trials (PubMed®)
- Hypnosis - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Inflammation - Randomized Controlled Trials (PubMed®)
- Inflammation - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Irritable Bowel Syndrome - Randomized Controlled Trials (PubMed®)
- Irritable Bowel Syndrome - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Kratom - Randomized Controlled Trials (PubMed®)
- Kratom - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Literature on Pediatric Immunization Controversy (PubMed®)
- Low Back Pain - Randomized Controlled Trials (PubMed®)
- Low Back Pain - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Lyme Disease - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Magnets and Pain - Randomized Controlled Trials (PubMed®)
- Magnets and Pain - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Massage - Randomized Controlled Trials (PubMed®)
- Massage - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Meditation - Randomized Controlled Trials (PubMed®)
- Meditation - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Menopausal Symptoms and Complementary Health Practices—Randomized Controlled Trials (PubMed®)
- Menopausal Symptoms and Complementary Health Practices—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Menopause - Randomized Controlled Trials (PubMed®)
- Menopause - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Mental Disorders - Randomized Controlled Trials (PubMed®)
- Mental Disorders - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Military Personnel - Randomized Controlled Trials (PubMed®)
- Military Personnel - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Milk Thistle - Herb-Drug Interactions (PubMed®)
- Milk Thistle - Randomized Controlled Trials (PubMed®)
- Milk Thistle - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Mind and Body Approaches for Stress—Randomized Controlled Trials (PubMed®)
- Mind and Body Approaches for Stress—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Mind and Body Practices for Fibromyalgia—Randomized Controlled Trials (PubMed®)
- Mind and Body Practices for Fibromyalgia—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Mind and Body Practices for Older Adults—Randomized Controlled Trials (PubMed®)
- Mind and Body Practices for Older Adults—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Multiple Sclerosis - Randomized Controlled Trials (PubMed®)
- Multiple Sclerosis and Complementary Health Approaches—Randomized Controlled Trials (PubMed®)
- Multiple Sclerosis and Complementary Health Approaches—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Musculoskeletal Inflammation and Natural Products—Randomized Controlled Trials (PubMed®)
- Music and Health - Randomized Controlled Trials (PubMed®)
- Music and Health - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Myth-Busting Popular Natural Products—Randomized Controlled Trials (PubMed®)
- Myth-Busting Popular Natural Products—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Natural Products Marketed for Disease Prevention and Wellness - Randomized Controlled Trials (PubMed®)
- Natural Products Marketed for Disease Prevention and Wellness - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Naturopathy - Randomized Controlled Trials (PubMed®)
- Naturopathy - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- NHIS2017 - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Omega-3 and Cardiovascular Diseases - Randomized Controlled Trials (PubMed®)
- Omega-3 and Cardiovascular Diseases - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Omega-3 Fatty Acids - Randomized Controlled Trials (PubMed®)
- Omega-3 Fatty Acids - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Omega-3 Fatty Acids and Prostate Cancer - Randomized Controlled Trials (PubMed®)
- Omega-3 Fatty Acids and Prostate Cancer - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Osteoarthritis - Randomized Controlled Trials (PubMed®)
- Osteoarthritis - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Pain - Randomized Controlled Trials (PubMed®)
- Pain - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Parkinson's Disease - Randomized Controlled Trials (PubMed®)
- Parkinson's Disease - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Pediatrics - Randomized Controlled Trials (PubMed®)
- Pediatrics - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Placebo Effect - Literature on Placebo Effect (PubMed®)
- Placebo Effect - Randomized Controlled Trials (PubMed®)
- Placebo Effect - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Post-Traumatic Stress Disorder - Randomized Controlled Trials (PubMed®)
- Post-Traumatic Stress Disorder - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Probiotics - Randomized Controlled Trials (PubMed®)
- Probiotics - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Qi Gong - Randomized Controlled Trials (PubMed®)
- Qi Gong - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Qigong—Randomized Controlled Trials (PubMed®)
- Qigong—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Reflexology - Randomized Controlled Trials (PubMed®)
- Reflexology - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Reiki - Randomized Controlled Trials (PubMed®)
- Reiki - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Reservatrol - Randomized Controlled Trials (PubMed®)
- Reservatrol - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Rheumatoid Arthritis - Randomized Controlled Trials (PubMed®)
- Rheumatoid Arthritis - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Safety and Complementary Therapies - Literature on Safety and Complementary Therapies (PubMed®)
- Saw Palmetto - Herb-Drug Interactions (PubMed®)
- Saw Palmetto - Randomized Controlled Trials (PubMed®)
- Saw Palmetto - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Seasonal Affective Disorder - Randomized Controlled Trials (PubMed®)
- Seasonal Affective Disorder - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Seasonal Allergies - Randomized Controlled Trials (PubMed®)
- Seasonal Allergies - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Seasonal Allergies and Complementary Health Approaches—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Sexual Enhancement - Randomized Controlled Trials (PubMed®)
- Sexual Enhancement - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Silymarin - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Silymarin - Randomized Controlled Trials (PubMed®)
- Skin Conditions - Randomized Controlled Trials (PubMed®)
- Skin Conditions - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Skin Conditions and Complementary Health Approaches—Randomized Controlled Trials (PubMed®)
- Skin Conditions and Complementary Health Approaches—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Sleep Disorders - Randomized Controlled Trials (PubMed®)
- Sleep Disorders - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Smoking Cessation - Randomized Controlled Trials (PubMed®)
- Smoking Cessation - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Soy - Herb-Drug Interactions (PubMed®)
- Soy - Randomized Controlled Trials (PubMed®)
- Soy - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Spinal Manipulation - Randomized Controlled Trials (PubMed®)
- Spinal Manipulation - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Spinal Manipulation and Low-Back Pain - Randomized Controlled Trials (PubMed®)
- Spinal Manipulation and Low-Back Pain - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- St. John's Wort - Herb-Drug Interactions (PubMed®)
- St. John's Wort - Randomized Controlled Trials (PubMed®)
- St. John's Wort - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- St. John’s Wort and Depression - Randomized Controlled Trials (PubMed®)
- St. John’s Wort and Depression - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- St. John’s Wort Herb-Drug Interactions - Herb-Drug Interactions (PubMed®)
- Stress - Randomized Controlled Trials (PubMed®)
- Stress - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Substance Use Disorders - Randomized Controlled Trials (PubMed®)
- Substance Use Disorders - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Tai Chi - Randomized Controlled Trials (PubMed®)
- Tai Chi - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Tea - Herb-Drug Interactions (PubMed®)
- Tea - Randomized Controlled Trials (PubMed®)
- Tea - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Traditional Chinese Medicine - Randomized Controlled Trials (PubMed®)
- Traditional Chinese Medicine - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Type 2 Diabetes and Dietary Supplements—Randomized Controlled Trials (PubMed®)
- Type 2 Diabetes and Dietary Supplements—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Urinary Tract Infections and Complementary Health Practices - Randomized Controlled Trials (PubMed®)
- Urinary Tract Infections and Complementary Health Practices - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Vitamin D - Randomized Controlled Trials (PubMed®)
- Vitamin D - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Weight Loss - Randomized Controlled Trials (PubMed®)
- Weight Loss - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Yoga for Health—Randomized Controlled Trials (PubMed®)
- Yoga for Health—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Yoga, Meditation, and Chiropractic—Randomized Controlled Trials (PubMed®)
- Yoga, Meditation, and Chiropractic—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Yoga—Randomized Controlled Trials (PubMed®)
- Yoga—Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Zinc for Common Colds - Randomized Controlled Trials (PubMed®)
- Zinc for Common Colds - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
- Research article
- Open access
- Published: 22 August 2024
A systematic review and meta-analysis of randomized trials of substituting soymilk for cow’s milk and intermediate cardiometabolic outcomes: understanding the impact of dairy alternatives in the transition to plant-based diets on cardiometabolic health
- M. N. Erlich 1 , 2 ,
- D. Ghidanac 1 , 2 ,
- S. Blanco Mejia 1 , 2 ,
- T. A. Khan 1 , 2 ,
- L. Chiavaroli 1 , 2 , 3 ,
- A. Zurbau 1 , 2 ,
- S. Ayoub-Charette 1 , 2 ,
- A. Almneni 4 ,
- M. Messina 5 ,
- L. A. Leiter 1 , 2 , 3 , 6 , 7 ,
- R. P. Bazinet 1 ,
- D. J. A. Jenkins 1 , 2 , 3 , 6 , 7 ,
- C. W. C. Kendall 1 , 2 , 8 &
- J. L. Sievenpiper 1 , 2 , 3 , 6 , 7
BMC Medicine volume 22 , Article number: 336 ( 2024 ) Cite this article
427 Accesses
65 Altmetric
Metrics details
Dietary guidelines recommend a shift to plant-based diets. Fortified soymilk, a prototypical plant protein food used in the transition to plant-based diets, usually contains added sugars to match the sweetness of cow’s milk and is classified as an ultra-processed food. Whether soymilk can replace minimally processed cow’s milk without the adverse cardiometabolic effects attributed to added sugars and ultra-processed foods remains unclear. We conducted a systematic review and meta-analysis of randomized controlled trials, to assess the effect of substituting soymilk for cow’s milk and its modification by added sugars (sweetened versus unsweetened) on intermediate cardiometabolic outcomes.
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched (through June 2024) for randomized controlled trials of ≥ 3 weeks in adults. Outcomes included established markers of blood lipids, glycemic control, blood pressure, inflammation, adiposity, renal disease, uric acid, and non-alcoholic fatty liver disease. Two independent reviewers extracted data and assessed risk of bias. The certainty of evidence was assessed using GRADE (Grading of Recommendations, Assessment, Development, and Evaluation). A sub-study of lactose versus sucrose outside of a dairy-like matrix was conducted to explore the role of sweetened soymilk which followed the same methodology.
Eligibility criteria were met by 17 trials ( n = 504 adults with a range of health statuses), assessing the effect of a median daily dose of 500 mL of soymilk (22 g soy protein and 17.2 g or 6.9 g/250 mL added sugars) in substitution for 500 mL of cow’s milk (24 g milk protein and 24 g or 12 g/250 mL total sugars as lactose) on 19 intermediate outcomes. The substitution of soymilk for cow’s milk resulted in moderate reductions in non-HDL-C (mean difference, − 0.26 mmol/L [95% confidence interval, − 0.43 to − 0.10]), systolic blood pressure (− 8.00 mmHg [− 14.89 to − 1.11]), and diastolic blood pressure (− 4.74 mmHg [− 9.17 to − 0.31]); small important reductions in LDL-C (− 0.19 mmol/L [− 0.29 to − 0.09]) and c-reactive protein (CRP) (− 0.82 mg/L [− 1.26 to − 0.37]); and trivial increases in HDL-C (0.05 mmol/L [0.00 to 0.09]). No other outcomes showed differences. There was no meaningful effect modification by added sugars across outcomes. The certainty of evidence was high for LDL-C and non-HDL-C; moderate for systolic blood pressure, diastolic blood pressure, CRP, and HDL-C; and generally moderate-to-low for all other outcomes. We could not conduct the sub-study of the effect of lactose versus added sugars, as no eligible trials could be identified.
Conclusions
Current evidence provides a good indication that replacing cow’s milk with soymilk (including sweetened soymilk) does not adversely affect established cardiometabolic risk factors and may result in advantages for blood lipids, blood pressure, and inflammation in adults with a mix of health statuses. The classification of plant-based dairy alternatives such as soymilk as ultra-processed may be misleading as it relates to their cardiometabolic effects and may need to be reconsidered in the transition to plant-based diets.
Trial registration
ClinicalTrials.gov identifier, NCT05637866.
Peer Review reports
Major dietary guidelines recommend a shift to plant-based diets for public and planetary health [ 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 ] , while recommending simultaneous reductions in ultra-processed foods [ 2 , 3 , 4 , 5 , 6 , 7 , 8 ]. The shift to plant-based diets has resulted in an explosion of dairy, meat, and egg alternatives with plant protein foods projected to reach almost 10% of the global protein market by 2030 [ 9 ]. Although these foods can aid in the transition to plant-based diets, food classification systems such as the World Health Organization (WHO)-endorsed NOVA classification system classify them as ultra-processed foods to be avoided [ 10 ].
Dairy alternatives are an important example of a food category at the crossroads of these competing recommendations. School milk programs provide > 150 million servings of cow’s milk to children worldwide [ 11 ]. These programs are in addition to the food service and procurement policies of public institutions such as schools, universities, hospitals, long-term care homes, and prisons. Many of these programs and policies do not allow for the free replacement of cow’s milk with nutrient-dense plant milks [ 12 , 13 ]. Although the Dietary Guidelines for Americans [ 1 ], Canada’s Food Guide [ 3 ], and several European food-based dietary guidelines [ 14 ] recognize fortified soymilk [ 1 ] as nutritionally equivalent to cow’s milk, school nutrition programs in the United States (US) [ 12 ] and Europe [ 13 ] only provide funding for cow’s milk. There is a bipartisan bill before the US congress to change this policy and provide funding for fortified soymilk [ 15 ]. A major barrier to the use of fortified soymilk is that it contains added sugars to match the sweetness of cow’s milk at a level which would disqualify it from meeting the Food and Drug Administration’s proposed definition of “healthy” [ 16 ] (although its total sugar content is usually ~ 60% less than that of cow’s milk given the higher sweetness intensity of sucrose vs lactose) [ 17 ] and is classified (irrespective of its sugar content) as an ultra-processed food to be avoided [ 10 , 18 ]. Cow’s milk, on the other hand, enjoys classification as a “healthy,” minimally processed food to be encouraged [ 10 , 18 ].
As industry innovates in response to the growing demand and policy makers develop public health nutrition policies and programs in response to the evolving dietary guidance for more plant-based diets, it is important to understand whether nutrient-dense ultra-processed plant protein foods can replace minimally processed dairy foods without the adverse cardiometabolic effects attributed to added sugars and ultra-processed foods. We conducted a systematic review and meta-analysis of randomized controlled trials of the effect of substituting soymilk for minimally processed cow’s milk and its modification by added sugars (sweetened versus unsweetened) on intermediate cardiometabolic outcomes as a basis for understanding the role of nutrient-dense ultra-processed plant protein foods in the transition to plant-based diets.
We followed the Cochrane Handbook for Systematic Reviews of Interventions to conduct this systematic review and meta-analysis and reported our results by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [ 19 , 20 ] (Additional file 1 : Table 1). To explore whether added sugars mediate any effects observed in sweetened soymilk studies, we conducted an additional systematic review and meta-analysis sub-study. This separate investigation followed the same protocol and methodology as our main study. It focused on controlled trials examining the impact of lactose in isocaloric comparisons with fructose-containing sugars (such as sucrose, high-fructose corn syrup [HFCS], or fructose) when not included in a dairy-like matrix, on all outcomes in the main study. The protocol is registered at ClinicalTrials.gov (NCT05637866).
Data sources and search strategy
We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials databases through June 2024. The detailed search strategies for the main study and sub-study were based on validated search terms [ 21 ] (Additional file 1 : Tables 2 and 4). Manual searches of the reference lists of included studies supplemented the systematic search.
Study selection
The main study included randomized controlled trials in human adults with any health status. Included trials had a study duration of ≥ 3 weeks and investigated the effects of soymilk compared with cow’s milk in energy matched conditions on intermediate cardiometabolic outcomes (Additional file 1 : Table 3). Trials that included other comparators that were not cow’s milk or had no viable outcome data were excluded. No restrictions were placed on language. For the sub-study, we included controlled trials involving adults of all health statuses that had a study duration of ≥ 3 weeks and investigated the effects of added sugars compared with lactose on the same intermediate cardiometabolic outcomes (Additional file 1 : Table 5).
Data extraction
A minimum of two investigators (ME, DG, SBM, AA) independently extracted relevant data from eligible studies. Extracted data included study design, sample size, sample characteristics (age, body mass index [BMI], sex, health status), intervention characteristics (soymilk volume, total sugars content, soy protein dose), control characteristics (cow’s milk volume, total sugars content, milk protein dose, milk fat content), baseline outcome levels, background diet, follow-up duration, setting, funding sources, and outcome data. The authors were contacted for missing outcome data when it was indicated that a relevant outcome was measured but not reported. Graphically presented data were extracted from figures using Plot Digitizer [ 22 ].
Outcomes for the main study and sub-study included blood lipids (low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], non-high-density lipoprotein cholesterol [non-HDL-C], triglycerides, and apolipoprotein B [ApoB]), glycemic control (hemoglobin A1c [HbA1c], fasting plasma glucose, 2-h postprandial glucose, fasting insulin, and plasma glucose area under the curve [PG-AUC]), blood pressure (systolic blood pressure and diastolic blood pressure), inflammation (c-reactive protein [CRP]), adiposity (body weight, BMI, body fat, and waist circumference), kidney function and structure (creatinine, creatinine clearance, glomerular filtration rate [GFR], estimated glomerular filtration rate [eGFR], albuminuria, and albumin-creatinine ratio [ACR]), uric acid, and non-alcoholic fatty liver disease (NAFLD) (intrahepatocellular lipid [IHCL], alanine transaminase [ALT], aspartate aminotransferase [AST], and fatty liver index).
Mean differences (MDs) between the intervention and control arm and respective standard errors were extracted for each trial. If these were not provided, they were derived from available data using published formulas [ 19 ]. Mean pairwise difference in change-from-baseline values were preferred over end values. When median data was provided, they were converted to mean data with corresponding variances using methods developed by McGrath et al. [ 23 ]. When no variance data was available, the standard deviation of the MDs was borrowed from a trial similar in size, participants, and nature of intervention. All disagreements were reconciled by consensus or with a senior reviewer (JLS).
Risk of bias assessment
Included studies were assessed for the risk of bias independently and in duplicate by at least two investigators (ME, DG, SBM, AA) using the Cochrane Risk of Bias (ROB) 2 Tool [ 24 ]. The assessment was performed across six domains of bias (randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias). Crossover studies were assessed for an additional domain of bias (risk of bias arising from period or carryover effects). The ROB for each domain was assessed as “low” (plausible bias unlikely to seriously alter the results), “high” (plausible bias that seriously weakens confidence in results), or “some concern” (plausible bias that raises some doubt about the results). Reviewer discrepancies were resolved by consensus or arbitration by a senior investigator (JLS).
Statistical analysis
STATA (version 17; StataCorp LP, College Station, TX) was used for all analyses for the main study and sub-study. The principal effect measures were the mean pair-wise differences in change from baseline (or alternatively, end differences) between the intervention arm providing the soymilk and the cow’s milk comparator/control arm in each trial (significance at P MD < 0.05). Results are reported as MDs with 95% confidence intervals (95% CI). As one of our primary research questions relates to the role of added sugars as a mediator in any observed differences between soymilk and cow’s milk, we stratified results by the presence of added sugars in the soymilk (sweetened versus unsweetened) and assessed effect modification by this variable on pooled estimates. Data were pooled using the generic inverse variance method with DerSimonian and Laird random effect models [ 25 ]. Fixed effects were used when less than five trials were available for an outcome [ 26 ]. A paired analysis was applied for crossover designs and for within-individual correlation coefficient between treatment of 0.5 as described by Elbourne et al. [ 27 , 28 ].
Heterogeneity was assessed using the Cochran’s Q statistic and quantified using the I 2 statistic, where I 2 ≥ 50% and P Q < 0.10 were used as evidence of substantial heterogeneity [ 19 ]. Potential sources of heterogeneity were explored using sensitivity analyses. Sensitivity analyses were done via two methods. We conducted an influence analysis by systematically removing one trial at a time and recalculating the overall effect estimate and heterogeneity. A trial was considered influential if its removal explained the substantial heterogeneity or altered the direction, magnitude, or significance of the summary estimate. To determine whether the overall summary estimates were robust to the use of an assumed correlation coefficient for crossover trials, we conducted a second sensitivity analysis by using correlation coefficients of 0.25 and 0.75. If ≥ 10 trials were available, meta-regression analyses were used to assess the significance of each subgroup categorically and when possible, continuously (significance at P < 0.05). A priori subgroup analyses included soy protein dose, follow-up duration, baseline outcome levels, comparator, design, age, health status, funding, and risk of bias.
If ≥ 6 trials are available [ 29 ], dose–response analyses were performed using meta-regression to assess linear (by generalized least squares trend (GLST) estimation models) and non-linear spline curve modeling (by MKSPLINE procedure) dose–response gradients (significance at P < 0.05).
If ≥ 10 studies were available, publication bias was assessed by inspection of contour-enhanced funnel plots and formal testing with Egger’s and Begg’s tests (significance at P < 0.10) [ 30 , 31 , 32 ]. If evidence of publication bias was suspected, the Duval and Tweedie trim-and-fill method was performed to adjust for funnel plot asymmetry by imputing missing study data and assess for small-study effects [ 33 ].
Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the certainty of evidence. The GRADE Handbook and GRADEpro V.3.2 software were used [ 34 , 35 ]. A minimum of two investigators (ME, DG, SBM) independently performed GRADE assessments for each outcome [ 36 ]. Discrepancies were resolved by consensus or arbitration by the senior author (JLS). The overall certainty of evidence was graded as either high, moderate, low, or very low. Randomized trials are initially graded as high by default and then downgraded or upgraded based on prespecified criteria. Reasons for downgrading the evidence included study limitations (risk of bias assessed by the Cochrane ROB Tool), inconsistency of results (substantial unexplained interstudy heterogeneity, I 2 > 50% and P Q < 0.10), indirectness of evidence (presence of factors that limit the generalizability of the results), imprecision (the 95% CI for effect estimates overlap with the MID for benefit or harm), and publication bias (evidence of small-study effects). The evidence was upgraded if a significant dose–response gradient was detected. We defined the importance of the magnitude of the pooled effect estimates using prespecified MIDs (Additional file 1 : Table 6) with GRADE guidance [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 ] according to five levels: very large (≥ 10 MID); large (≥ 5 MID); moderate (≥ 2 MID); small important (≥ 1 MID); and trivial/unimportant (< 1 MID) effects.
Search results
Figure 1 in Appendix shows the flow of the literature for the main analysis. We identified 522 reports through database and manual searches. A total of 17 reports [ 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 ] met the inclusion criteria and contained data for LDL (10 trials, n = 312), HDL-C (8 trials, n = 271), non-HDL-C (7 trials, n = 243), triglycerides (9 trials, n = 278), HbA1c (1 trial, n = 25), fasting plasma glucose (5 trials, n = 147), 2-h plasma glucose (1 trial, n = 28), fasting insulin (4 trials, n = 119), systolic blood pressure (5 trials, n = 158), diastolic blood pressure (5 trials, n = 158), CRP (5 trials, n = 147), body weight (6 trials, n = 163), BMI (6 trials, n = 173), body fat (1 trial, n = 43), waist circumference (3 trials, n = 90), creatinine (1 trial, n = 25), eGFR (1 trial, n = 25), ALT (1 trial, n = 24), and AST (1 trial, n = 24) involving 504 participants. No trials were available for ApoB, PG-AUC, creatinine clearance, eGFR, albuminuria, ACR, uric acid, IHCL, or fatty liver index.
Additional file 1 : Fig. 1 shows the flow of literature for the sub-study. We identified 1010 reports through database and manual searches. After excluding 305 duplicates, a total of 705 reports were reviewed by title and abstract. No reports met the inclusion criteria and therefore no data was available for analysis.
Trial characteristics
Table 1 shows the characteristics of the included trials. The trials were conducted in a variety of locations, with most conducted in Iran (7/17 trials, 41%), followed by the US (3/17 trials, 18%), Italy (2/17 trials, 12%), Brazil (1/17 trials, 6%), Scotland (1/17 trials, 6%), Sweden (1/17 trials, 6%), Spain (1/17 trials, 6%), and Australia (1/17 trials, 6%). All trials took place in outpatient settings (17/17, 100%). The median trial size was 25 participants (range, 7–60 participants). The median age of the participants was 48.5 years (range, 20–70 years) and the median BMI was 27.9 kg/m 2 (range, 20–31.1 kg/m 2 ). The trials included participants with hypercholesterolemia (4/17 trials, 25%), overweight or obesity (4/17 trials, 25%), type 2 diabetes (2/17 trials, 12%), hypertension (1/17 trials, 6%), rheumatoid arthritis (1/17 trials, 6%), or were healthy (3/17 trials, 18%) or post-menopausal (2/17 trials, 12%). Both trials with crossover design (10/17 trials, 59%) and parallel design (7/17 trials, 41%) were included. The intervention included sweetened (11/17 trials, 65%) and unsweetened (6/17 trials, 35%) soymilk.
The median soymilk dose was 500 mL/day (range, 240–1000 mL/day) with a median soy protein of 22 g/day (range, 2.5–70 g/day) or 6.6 g/250 mL (range, 2.6–35 g/250 mL) and median total (added) sugars of 17.2 g/day (range, 4.0–32 g/day) or 6.9 g/250 mL (range, 1–16 g/250 mL) in the sweetened soymilk. The comparators included skim (0% milk fat) (2/17 trials, 12%), low-fat (1% milk fat) (4/17 trials, 24%), reduced fat (1.5–2.5% milk fat) (7/17 trials, 41%), and whole (3% milk fat) (1/17 trials, 6%) cow’s milk. Three trials did not report the milk fat content of cow’s milk used. The median cow’s milk dose was 500 mL/day (range, 236–1000 mL/day) with a median milk protein of 24 g/day (range, 3.3–70 g/day) or 8.3 g/250 mL (range, 3.4–35 g/250 mL) and median total (lactose) sugars of 24 g/day (range, 11.5–49.2 g/day) or 12 g/250 mL (range, 10.8–12.8 g/250 mL). The median study duration was 4 weeks (range, 4–16 weeks). The trials received funding from industry (1/17 trials, 6%), agency (8/17 trials, 47%), both industry and agency (4/16 trials, 25%), or they did not report the funding source (4/17 trials, 24%).
Additional file 1 : Fig. 2 shows the ROB assessments of the included trials. Two trials were assessed as having some concerns from period or carryover effects: Bricarello et al. [ 53 ] and Steele [ 67 ]. All other trials were judged as having an overall low risk of bias. There was no evidence of serious risk of bias across the included trials.
Markers of blood lipids
Figure 2 and Additional file 1 : Figs. 3–6 show the effect of substituting soymilk for cow’s milk on markers of blood lipids. The substitution resulted in a small important reduction in LDL-C (10 trials; MD: − 0.19 mmol/L; 95% CI: − 0.29 to − 0.09 mmol/L; P MD < 0.001; no heterogeneity: I 2 = 0.0%; P Q = 0.823), a trivial increase in HDL-C (8 trials; MD: 0.05 mmol/L; 95% CI: 0.00 to 0.09 mmol/L; P MD = 0.036; no heterogeneity: I 2 = 0.0%; P Q = 0.053), a moderate reduction in non-HDL-C (7 trials; MD: − 0.26 mmol/L; 95% CI: − 0.43 to − 0.10 mmol/L; P MD = 0.002; no heterogeneity: I 2 = 0.0%; P Q = 0.977), and no effect on triglycerides. There were no interactions by added sugars in soymilk for any blood lipid markers ( P = 0.49–0.821).

Markers of glycemic control
Figure 2 and Additional file 1 : Figs. 7–10 show the effect of substituting soymilk for cow’s milk on markers of glycemic control. The substitution had no effect on HbA1c, fasting plasma glucose, 2-h plasma glucose, or fasting insulin. There was no interaction by added sugars in soymilk for fasting plasma glucose ( P = 0.747) but there was an interaction for fasting insulin ( P = 0.026), where a lack of effect remained in both groups with neither the sweetened soymilk (non-significant increasing effect) nor the unsweetened soymilk (non-significant decreasing effect) showing an effect on fasting insulin. We could not assess this interaction for HbA1c or 2-h plasma glucose, as there was only one trial available for each outcome.
Blood pressure
Figure 2 and Additional file 1 : Figs. 11 and 12 show the effect of substituting soymilk for cow’s milk on blood pressure. The substitution resulted in a moderate reduction in both systolic blood pressure (5 trials; MD: − 8.00 mmHg; 95% CI: − 14.89 to − 1.11 mmHg; P MD = 0.023; substantial heterogeneity: I 2 = 86.89%; P Q ≤ 0.001) and diastolic blood pressure (5 trials; MD: − 4.74 mmHg; 95% CI: − 9.17 to − 0.31 mmHg; P MD = 0.036; substantial heterogeneity: I 2 = 77.3%; P Q = 0.001). There were no interactions by added sugars in soymilk for blood pressure ( P = 0.747 and 0.964).
Markers of inflammation
Figure 2 and Additional file 1 : Fig. 13 show the effect of substituting soymilk for cow’s milk on markers of inflammation. The substitution resulted in a small important reduction in CRP (5 trials; MD: − 0.81 mg/dL; 95% CI: − 1.26 to − 0.37 mg/dL; P MD = < 0.001; no heterogeneity: I 2 = 0.0%; P Q = 0.814). There was no interaction by added sugars in soymilk for CRP ( P = 0.275).
Markers of adiposity
Figure 2 and Additional file 1 : Figs. 14–17 show the effect of substituting soymilk for cow’s milk on markers of adiposity. The substitution had no effect on body weight, BMI, body fat, or waist circumference. There were no interactions by added sugars in soymilk for any adiposity outcome ( P = 0.664–0.733).
Markers of kidney function
Figure 2 and Additional file 1 : Figs. 18 and 19 show the effect of substituting soymilk for cow’s milk on markers of kidney function. The substitution had no effect on creatinine or eGFR. We could not assess the interaction by added sugars in soymilk for creatinine or eGFR, as there was only one trial available for each outcome which included soymilk without added sugars.
Markers of NAFLD
Figure 2 and Additional file 1 : Figs. 20 and 21 show the effect of substituting soymilk for cow’s milk on markers of NAFLD. The substitution had no effect on ALT or AST. We could not assess heterogeneity or the interaction by added sugars in soymilk for ALT or AST, as there was only one trial available for each outcome which included soymilk without added sugars.
Sensitivity analysis
Additional file 1 : Figs. 22–33 present the influence analyses across all outcomes. The removal of Bricarello et al. [ 53 ] or Steele [ 67 ] each resulted in loss of significant effect for HDL-C. The removal of Onning et al. [ 62 ] or Steele [ 67 ] each resulted in a partial explanation of heterogeneity for triglycerides. The removal of Hasanpour et al. [ 56 ] explained the heterogeneity for fasting insulin. The removal of Keshavarz et al. [ 57 ] or Miraghajani et al. [ 59 ] each resulted in a loss of significant effect for systolic blood pressure and the removal of Rivas et al. [ 63 ] resulted in a partial explanation of the heterogeneity for systolic blood pressure. The removal of Hasanpour et al. [ 56 ], Keshavarz et al. [ 57 ], Miraghajani et al. [ 59 ], or Rivas et al. [ 63 ] each resulted in a loss of significant effect for diastolic blood pressure and the removal of Rivas et al. [ 63 ] resulted in a partial explanation of heterogeneity for diastolic blood pressure. The removal of Mohammad-Shahi et al. [ 58 ] resulted in loss of significant effect for CRP.
Additional file 1 : Table 8 shows the sensitivity analyses for the different correlation coefficients (0.25 and 0.75) used in paired analyses of crossover trials for all outcomes. The different correlation coefficients did not alter the direction, magnitude, or significance of the effect or evidence for heterogeneity, with the following exceptions: loss of significance for the effect of the substitution on HDL-C (8 trials; MD: 0.04 mmol/L; 95% CI: − 0.10 to 0.01 mmol/L; P MD = 0.107; I 2 = 0.0%; P Q = 0.670) with the use of 0.25 and (8 trials; MD: 0.05 mmol/L; 95% CI: − 0.10 to 0.01 mmol/L; P MD = 0.089; I 2 = 0.0%; P Q = 0.640) with the use of 0.75.
Subgroup analyses
Additional file 1 : Figs. 34–36 present the subgroup analyses and continuous meta-regression analyses for LDL-C. Subgroup analysis was not conducted for any other outcome as there were < 10 trials included. There was no significant effect modification by health status, BMI, age, comparator, baseline LDL-C, study design, follow-up duration, funding source, dose of soy protein, or risk of bias for LDL-C. However, there were tendencies towards a greater reduction in LDL-C by point estimates in groups with certain health statuses (hypercholesterolemic and overweight/obesity), a higher baseline LDL-C, and a higher soy protein dose (> 25 g/day).
Dose–response analyses
Additional file 1 : Figs. 37–42 present linear and non-linear dose–response analyses for LDL-C, HDL-C, non-HDL-C, triglycerides, body weight, and BMI. There was no dose–response seen for the effect of substituting soymilk for cow’s milk, with the exception of a positive linear dose–response for triglycerides ( P linear = 0.038). We did not downgrade the certainty of evidence as the greater reduction in triglycerides seen at lower doses of soy protein was lost at higher doses. There were no dose–response analyses performed for the remaining outcomes because there were < 6 trials available for each.
Publication bias assessment
Additional file 1 : Fig. 43 presents the contour-enhanced funnel plot for assessment of publication bias for LDL-C. There was no asymmetry at the visual inspection and no evidence (Begg’s test = 0.721, Egger’s test = 0.856) of funnel plot asymmetry for LDL-C. No other publication bias analyses could be performed as there were < 10 trials available for each.
Adverse events and acceptability
Additional file 1 : Table 9 shows the reported adverse events and acceptability of study beverages. Adverse events were reported in nine trials. In one trial by Gardner et al. [ 55 ], one participant experienced a recurrence of a cancer; however, it was considered to be unrelated to the short-term consumption of the study milks. Three trials (Miraghajani et al., Hasanpour et al., and Mohammad-Shahi, et al.) [ 56 , 58 , 59 ] reported one to two withdrawals due to digestive difficulties related to soymilk consumption. Two trials (Sirtori et al. 1999 and 2002) [ 65 , 66 ] reported one or more participants with digestive difficulties related to cow’s milk consumption. Two trials (Nourieh et al. and Keshavarz et al.) [ 57 , 61 ] each reported two participant withdrawals related to digestive problems that were not specific to either study beverage. Of these, four trials indicated that most participants found the soymilk and cow’s milk acceptable and tolerable. One trial, by Onning et al. [ 62 ], incorporated a sensory evaluation of appearance, consistency, flavor, and overall impression, which showed declining scores for both types of milk over the 3-week test period.
GRADE assessment
Additional file 1 : Table 10 presents the GRADE assessment. The certainty of evidence for the effect of substituting soymilk for cow’s milk was high for LDL-C, non-HDL-C, fasting plasma glucose, and waist circumference. The certainty of evidence was moderate for HDL-C, triglycerides, fasting insulin, systolic blood pressure, diastolic blood pressure, CRP, body weight, and BMI owing to a downgrade for imprecision of the pooled effect estimates and was moderate for body fat owing to a downgrade for indirectness. The certainty of evidence was low for HbA1c, 2-h plasma glucose, creatinine, eGFR, ALT, and AST owing to downgrades for indirectness and imprecision.
We conducted a systematic review and meta-analysis of 17 trials that examined the effect of substituting soymilk (median dose of 22 g/day or 6.6 g/250 mL serving of soy protein per day and 17.2 g/day or 6.9 g/250 mL of total [added] sugars in the sweetened soymilk) for cow’s milk (median dose of 24 g/day or 8.3 g/250 mL of milk protein and 24 g/day or 12 g/250 mL of total sugars [lactose]) and its modification by added sugars (sweetened versus unsweetened soymilk) on 19 intermediate cardiometabolic outcomes over a median follow-up period of 4 weeks in adults of varying health status. The substitution of soymilk for cows’ milk led to moderate reductions in non-HDL-C (− 0.26 mmol/L or ~ − 7%) and systolic blood pressure (− 8.00 mmHg) and diastolic blood pressure (− 4.74 mmHg); small important reductions in LDL-C (− 0.19 mmol/L or ~ − 6%) and CRP (− 0.81 mg/L or ~ 22%); and a trivial increase in HDL-C (0.05 mmol/L or ~ 4%), with no adverse effects on other intermediate cardiometabolic outcomes. There was no meaningful interaction by added sugars in soymilk, with sweetened and unsweetened soymilk showing similar effects across outcomes. There was no dose–response relationship seen across the outcomes for which dose–response analyses were performed.
Findings in relation to the literature
Our findings agree with previous evidence syntheses of soy. Regulatory authorities such as the United States Food and Drug Administration (FDA) and Health Canada have conducted comprehensive evaluations of the randomized controlled trials of the effect of soy protein from different sources on total-C and LDL-C, resulting in approved health claims for soy protein (based on an intake of 25 g/day of soy protein irrespective of source) for cholesterol reduction [ 68 ] and coronary heart disease risk reduction [ 69 ]. Updated systematic reviews and meta-analyses of the 46 randomized controlled trials included in the re-evaluation of the FDA health claim [ 70 ] showed reductions in LDL-C of − 3.2% [ 71 ]. This reduction has been stable since the health claim was first approved in 1999 [ 72 ] and is smaller but consistent with our findings specifically for soymilk. No increase in HDL-C, however, was detected. Previous systematic reviews and meta-analyses of randomized controlled trials of soy protein and soy isoflavones have also shown significant but smaller reductions in systolic blood pressure (1.70 mmHg) and diastolic blood pressure (− 1.27 mmHg) [ 73 ] than was found in the current analysis. These reductions in LDL-C and blood pressure are further supported by reductions in clinical events with updated pooled analyses of prospective cohort studies showing that legumes including soy are associated with reduced incidence of total cardiovascular disease and coronary heart disease [ 74 ].
Systematic reviews and meta-analyses that specifically isolated the effect of soymilk (as a single food matrix) in its intended substitution for cow’s milk are lacking. Sohouli and coworkers [ 75 ] conducted a systematic review and meta-analysis of 18 randomized controlled trials in 665 individuals of varying health status that assessed the effect of soymilk in comparison with a mix of comparators on intermediate cardiometabolic outcomes but did not isolate its substitution with cow’s milk. This synthesis showed similar improvements in LDL-C (− 0.24 mmol/L), systolic blood pressure (− 7.38 mmHg), diastolic blood pressure (− 4.36 mmHg), and CRP (− 1.07, mg/L), while also showing reductions in waist circumference and TNF-α [ 75 ]. The substitution of legumes that includes soy for various animal protein sources and more specifically legumes/nuts (the only exposure available) for dairy in syntheses of prospective cohort studies has also shown reductions in incident total cardiovascular disease and all-cause mortality [ 76 ].
Indirect evidence from dietary patterns that contain soy foods including soymilk in substitution for different animal sources of protein including cow’s milk further supports our findings. Systematic reviews and meta-analyses of randomized trials of the Portfolio diet and vegetarian and vegan dietary patterns have shown additive reductions in LDL-C, non-HDL-C, blood pressure, and CRP when soy foods including soymilk are combined with other foods that target these same intermediate risk factors with displacement of different animal sources of protein including cow’s milk [ 77 , 78 ]. These reductions have also been shown to translate to reductions in clinical events with systematic reviews and meta-analyses of prospective cohort studies showing that adherence to these dietary patterns is associated with reductions in incident coronary heart disease, total cardiovascular disease, and all-cause mortality [ 79 , 80 , 81 ].
Potential mechanisms of action
The potential mechanism mediating the effects of soy remains unclear. Specific components within the soy food matrix, including soy protein and phytochemicals like isoflavones [ 82 ], have been implicated. The well-established lipid-lowering effect of soy [ 72 ] may be attributed to the 7S globulin fraction of soy protein, which exerts its primary action by upregulating LDL-C receptors predominantly within the liver, thereby augmenting the clearance of LDL-C from circulation [ 82 ]. The isoflavone, fiber, fatty acids, and anti-nutrient components may also exert some mediation [ 83 ]. The reduction in blood pressure has been most linked to the soy isoflavones [ 83 ]. There is evidence that soy isoflavones may modulate the renin–angiotensin–aldosterone system (RAAS), with the capacity to inhibit the production of angiotensin II and aldosterone, thereby contributing to the regulation of blood pressure [ 73 ]. Another blood pressure lowering mechanism may involve the ability of soy isoflavones to enhance endothelial function by mitigating oxidative stress and inflammation, consequently promoting the release of the relaxing factor nitric oxide (NO) [ 73 ]. This potential mechanism of isoflavones may also explain the reductions seen in inflammation.
Strengths and limitations
Our evidence synthesis had several strengths. First, we completed a comprehensive and reproducible systematic search and selection process of the available literature examining the effect of substituting soymilk for cow’s milk on intermediate cardiometabolic outcomes. Second, we synthesized the totality of available evidence from a large body of randomized controlled trials, which gives the greatest protection against systematic error. Third, we included an extensive and comprehensive list of outcomes to fully capture the impact of soymilk on cardiometabolic health. Fourth, we only included randomized controlled trials that compared soymilk to cow’s milk directly, to increase the specificity of our conclusion. Finally, we included a GRADE assessment to explore the certainty of available evidence.
There were also several limitations. First, we could not conduct the sub-study of the effect of lactose versus added sugars outside of a dairy-like matrix, as no eligible trials could be identified. Although this analysis is important for isolating the effect of added sugars as a mediator of any adverse effects, we did not observe any meaningful interaction by added sugars in soymilk. Second, there was serious imprecision in the pooled estimates across many of the outcomes with the 95% confidence intervals overlapping the MID in each case, with the exception of LDL-C, non-HDL-C, fasting plasma glucose, and waist circumference. The certainty of evidence for HDL-C, triglycerides, HbA1c, fasting plasma glucose, 2-h plasma glucose, fasting insulin, systolic blood pressure, diastolic blood pressure, CRP, body weight, BMI, body fat, creatinine, eGFR, ALT, and AST was downgraded for this reason. Third, there was evidence of indirectness related to insufficient trials for HbA1c, 2-h plasma glucose, creatinine, eGFR, ALT, and AST, which limits generalizability. Each outcome with data from only 1 trial was downgraded for this reason. Another source of indirectness could be the median follow-up duration of 4 weeks (range, 4–16 weeks). This time frame may be sufficient for observing certain effects, but other outcomes may require a longer period to manifest changes. Despite acknowledging this variation in response time among different outcomes, we did not further downgrade for this aspect of indirectness. Instead, we tailored our conclusions to reflect short-to-moderate term effects. Finally, although publication bias was not suspected, we were only able to make this assessment for LDL-C, as there were < 10 trials for all other outcomes.
Considering these strengths and limitations, we assessed the certainty of evidence as high for LDL-C and non-HDL-C; moderate for systolic blood pressure, diastolic blood pressure, CRP, and HDL-C; and moderate-to-low for all outcomes where significant effects were not observed.
Implications
This work has important implications for plant protein foods in the recommended shift to more plant-based diets. Major international dietary guidelines in the US [ 1 ], Canada [ 3 ], and Europe [ 4 , 5 , 6 ] recommend fortified soymilk as the only suitable replacement for cow’s milk. Our findings support this recommendation showing soymilk including sweetened soymilk (up to 7 g added sugars per 250 mL) does not have any adverse effects compared with cow’s milk across 19 intermediate cardiometabolic outcomes with benefits for lipids, blood pressure, and inflammation. This evidence suggests that it may be misleading as it relates to their cardiometabolic effects to classify fortified soymilk as an ultra-processed food to be avoided while classifying cow’s milk as a minimally processed food to be encouraged (based on the WHO-endorsed NOVA classification system [ 10 ]). It also suggests that it may be misleading not to allow fortified soymilk that is sweetened with small amounts of sugars to be classified as “healthy” (based on the FDA’s new proposed definition that only permits this claim on products with added sugars ≤ 2.5 g or 5% daily value (DV) per 250 mL serving [ 16 ]). The proposed FDA criteria would prevent this claim on soymilk products designed to be iso-sweet analogs of cow’s milk (in which 5 g or 10% daily value [DV] of added sugars from sucrose in soymilk is equivalent to the 12 g of lactose in cow’s milk per 250 mL serving, as sucrose is 1.4 sweeter than lactose [ 17 ]). To prevent confusion, policy makers may want to exempt fortified soymilk from classification as an ultra-processed food and allow added sugars up to 10% DV for the definition of “healthy,” as has been proposed by the FDA for sodium and saturated fat in dairy products (including soy-based dairy alternatives) to account for accepted processing and preservation methods [ 16 ]. These policy considerations would balance the need to limit nutrient-poor energy-dense foods with the need to promote nutrient-dense foods like fortified soymilk in the shift to healthy plant-based diets.
In conclusion, the evidence provides a good indication that substituting either sweetened or unsweetened soymilk for cow’s milk in adults with varying health statuses does not have the adverse effects on intermediate cardiometabolic outcomes attributed to added sugars and ultra-processed foods in the short-to-moderate term. There appear even to be advantages with small to moderate reductions in established markers of blood lipids (LDL-C, non-HDL-C) that are in line with approved health claims for cholesterol and coronary heart disease risk reduction, as well as small to moderate reductions in blood pressure and inflammation (CRP). Sources of uncertainty include imprecision and indirectness in several of the estimates. There remains a need for more well-powered randomized controlled trials of the effect of substituting soymilk for cow’s milk on less studied intermediate cardiometabolic outcomes, especially established markers of glycemic control, kidney structure and function, and NAFLD. There is also a need for trials comparing lactose versus added sugars outside of a dairy-like matrix to understand better the role of added sugars at different levels in substitution for lactose across outcomes. In the meantime, our findings support the use of fortified soymilk with up to 7 g added sugars per 250 mL as a suitable replacement for cow’s milk and suggest that its classification as ultra-processed and/or not healthy based on small amounts of added sugars may be misleading and need to be reconsidered to facilitate the recommended transition to plant-based diets.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1 : information files.
Abbreviations
Grading of Recommendations, Assessment, Development, and Evaluation
Non-high-density lipoprotein cholesterol
Low-density lipoprotein cholesterol
C-reactive protein
High-density lipoprotein cholesterol
World Health Organization
United States
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
High-fructose corn syrup
Body mass index
Apolipoprotein B
Hemoglobin A1c
Plasma glucose area under the curve
Glomerular filtration rate
Estimated glomerular filtration rate
Albumin-creatinine ratio
Non-alcoholic fatty liver disease
Intrahepatocellular lipid
Alanine transaminase
Aspartate aminotransferase
Mean difference
Risk of bias
95% Confidence interval
Generalized least squares trend
Food and Drug Administration
Tumor necrosis factor alpha
Renin-angiotensin-aldosterone system
Nitric oxide
Daily value
Dietary guidelines for Americans, 2020–2025. 2020 [9:[Available from: www.dietaryguidelines.gov .
Canada, Health. Canada’s Food Guide. Ottawa; 2019. https://food-guide.canada.ca/en/ .
Canada’s food guide Ottawa 2019 [Available from: https://food-guide.canada.ca/en/ .
Blomhoff R, Andersen R, Arnesen EK, Christensen JJ, Eneroth H, Erkkola M, Gudanaviciene I, Halldórsson ÞI, Höyer-Lund A, Lemming EW. Nordic nutrition recommendations 2023: integrating environmental aspects. Nordisk Ministerråd; 2023.
García EL, Lesmes IB, Perales AD, Arribas VM, del Puy Portillo Baquedano M, Velasco AMR, Salvo UF, Romero LT, Porcel FBO, Laín SA. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on sustainable dietary and physical activity recommendations for the Spanish population. Wiley Online Library; 2023. Report No.: 2940–1399.
Brink E, van Rossum C, Postma-Smeets A, Stafleu A, Wolvers D, van Dooren C, et al. Development of healthy and sustainable food-based dietary guidelines for the Netherlands. Public Health Nutr. 2019;22(13):2419–35.
Article PubMed PubMed Central Google Scholar
Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM, et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472–87.
Article PubMed Google Scholar
Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. The lancet. 2019;393(10170):447–92.
Article Google Scholar
Bartashus J, Srinivasan G. Plant-based foods poised for explosive growth. Bloomberg Intelligence. 2021.
Monteiro CA, Cannon G, Lawrence M, Costa Louzada Md, Pereira Machado P. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome: FAO; 2019. p. 48.
International Dairy Federation. The contribution of school milk programmes to the nutrition of children worldwide. Brussels: Belgium; 2020.
Google Scholar
USDA Food and Nutrition Service. Special Milk Program [Available from: https://www.fns.usda.gov/smp/special-milk-program .
The European Parliament. European Parliament resolution of 9 May 2023 on the implementation of the school scheme [Available from: https://www.europarl.europa.eu/doceo/document/TA-9-2023-0135_EN.html .
European Commission. Summary of FBDG recommendations for milk and dairy products for the EU, Iceland, Norway, Switzerland and the United Kingdom. [Available from: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/food-based-dietary-guidelines-europe-table-7_en .
Addressing Digestive Distress in Stomachs of Our Youth (ADD SOY) Act, House of Representatives, 1st Sess.; 2023. https://troycarter.house.gov/sites/evo-subsites/troycarter.house.gov/files/evo-media-document/add-soy-act.pdf .
Food and Drug Administration. Food labeling: nutrient content claims; definition of term “healthy”. In: Department of Health and Human Services (HHS); 2022. https://www.federalregister.gov/documents/2022/09/29/2022-20975/food-labeling-nutrient-content-claims-definition-of-term-healthy .
Helstad S. Chapter 20 - corn sweeteners. In: Serna-Saldivar SO, editor. Corn. 3rd ed. Oxford: AACC International Press; 2019. p. 551–91.
Chapter Google Scholar
Messina M, Sievenpiper JL, Williamson P, Kiel J, Erdman JW. Perspective: soy-based meat and dairy alternatives, despite classification as ultra-processed foods, deliver high-quality nutrition on par with unprocessed or minimally processed animal-based counterparts. Adv Nutr. 2022;13(3):726–38.
Article PubMed PubMed Central CAS Google Scholar
Higgins J, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.2. 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
BMJ Best Practice. Search strategies [Available from: https://bestpractice.bmj.com/info/toolkit/learn-ebm/study-design-search-filters/ .
Rohatgi A. WebPlotDigitizer 4.6; 2022. https://automeris.io/WebPlotDigitizer/ .
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):2520–37.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Article PubMed CAS Google Scholar
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207.
Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9.
Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within-arm correlation imputation in trials of continuous outcomes. 2013.
Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64(11):1187–97.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook. Grading of Recommendations Assessment, Development and Evaluation, Grade Working Group. 2013.
McMaster University and Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. gradepro.org .
Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66(2):140–50.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283–93.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303–10.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–302.
Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277–82.
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66(2):158–72.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6.
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15.
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173–83.
Kaminski-Hartenthaler A, Gartlehner G, Kien C, Meerpohl JJ, Langer G, Perleth M, et al. GRADE-Leitlinien: 11. Gesamtbeurteilung des Vertrauens in Effektschätzer für einen einzelnen Studienendpunkt und für alle Endpunkte. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen. 2013;107(9):638–45.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, et al. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. J Clin Epidemiol. 2016;74:19–27.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28–39.
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–35.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH, Group, Cochrane GRADEing Methods and Group, the Cochrane Statistical Methods. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence. Cochrane handbook for systematic reviews of interventions. 2019. p. 375–402.
Azadbakht L, Nurbakhsh S. Effect of soy drink replacement in a weight reducing diet on anthropometric values and blood pressure among overweight and obese female youths. Asia Pac J Clin Nutr. 2011;20(3):383–9.
PubMed CAS Google Scholar
Beavers KM, Serra MC, Beavers DP, Cooke MB, Willoughby DS. Soymilk supplementation does not alter plasma markers of inflammation and oxidative stress in postmenopausal women. Nutr Res. 2009;29(9):616–22.
Bricarello LP, Kasinski N, Bertolami MC, Faludi A, Pinto LA, Relvas WG, et al. Comparison between the effects of soy milk and non-fat cow milk on lipid profile and lipid peroxidation in patients with primary hypercholesterolemia. Nutrition. 2004;20(2):200–4.
Faghih S, Hedayati M, Abadi A, Kimiagar M. Comparison of the effects of cow’s milk, fortified soy milk, and calcium supplement on plasma adipocytokines in overweight and obese women. Iranian Journal of Endocrinology and Metabolism. 2009;11(6):692–8.
Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: a randomized trial. J Am Coll Nutr. 2007;26(6):669–77.
Hasanpour A, Babajafari S, Mazloomi SM, Shams M. The effects of soymilk plus probiotics supplementation on cardiovascular risk factors in patients with type 2 diabetes mellitus: a randomized clinical trial. BMC Endocr Disord. 2023;23(1):36.
Keshavarz SA, Nourieh Z, Attar MJ, Azadbakht L. Effect of soymilk consumption on waist circumference and cardiovascular risks among overweight and obese female adults. Int J Prev Med. 2012;3(11):798–805.
PubMed PubMed Central Google Scholar
Mohammad-Shahi M, Mowla K, Haidari F, Zarei M, Choghakhori R. Soy milk consumption, markers of inflammation and oxidative stress in women with rheumatoid arthritis: a randomised cross-over clinical trial. Nutr Diet. 2016;73(2):139–45.
Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35(10):1981–5.
Mitchell JH, Collins AR. Effects of a soy milk supplement on plasma cholesterol levels and oxidative DNA damage in men—a pilot study. Eur J Nutr. 1999;38(3):143–8.
Nourieh Z, Keshavarz SA, Attar MJH, Azadbakht L. Effects of soy milk consumption on inflammatory markers and lipid profiles among non-menopausal overweight and obese female adults. Int J Prev Med. 2012;3:798.
Onning G, Akesson B, Oste R, Lundquist I. Effects of consumption of oat milk, soya milk, or cow’s milk on plasma lipids and antioxidative capacity in healthy subjects. Ann Nutr Metab. 1998;42(4):211–20.
Rivas M, Garay RP, Escanero JF, Cia P Jr, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr. 2002;132(7):1900–2.
Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 2006;83(5):1118–25.
Sirtori CR, Pazzucconi F, Colombo L, Battistin P, Bondioli A, Descheemaeker K. Double-blind study of the addition of high-protein soya milk v. cows’ milk to the diet of patients with severe hypercholesterolaemia and resistance to or intolerance of statins. Br J Nutr. 1999;82(2):91–6.
Sirtori CR, Bosisio R, Pazzucconi F, Bondioli A, Gatti E, Lovati MR, et al. Soy milk with a high glycitein content does not reduce low-density lipoprotein cholesterolemia in type II hypercholesterolemic patients. Ann Nutr Metab. 2002;46(2):88–92.
Steele M. Effect on serum cholesterol levels of substituting milk with a soya beverage. Aust J Nutr Diet. 1992;49(1):24–8.
Summary of Health Canada’s assessment of a health claim about soy protein and cholesterol lowering Ottawa: Health Canada; 2015 [Available from: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/summary-assessment-health-claim-about-protein-cholesterol-lowering.html .
Food and Drug Administration. Food labeling health claims; soy protein and coronary heart disease. Fed Regist. 1999;64:57699–733.
Food and Drug Administration. Food labeling health claims; soy protein and coronary heart disease. Fed Regist. 2017;82:50324–46.
Blanco Mejia S, Messina M, Li SS, Viguiliouk E, Chiavaroli L, Khan TA, et al. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J Nutr. 2019;149(6):968–81.
Jenkins DJA, Blanco Mejia S, Chiavaroli L, Viguiliouk E, Li SS, Kendall CWC, et al. Cumulative meta-analysis of the soy effect over time. J Am Heart Assoc. 2019;8(13):e012458.
Mosallanezhad Z, Mahmoodi M, Ranjbar S, Hosseini R, Clark CCT, Carson-Chahhoud K, et al. Soy intake is associated with lowering blood pressure in adults: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Complement Ther Med. 2021;59:102692.
Viguiliouk E, Glenn AJ, Nishi SK, Chiavaroli L, Seider M, Khan T, et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv Nutr. 2019;10(Suppl_4):S308–19.
Sohouli MH, Lari A, Fatahi S, Shidfar F, Găman M-A, Guimaraes NS, et al. Impact of soy milk consumption on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Journal of Functional Foods. 2021;83:104499.
Neuenschwander M, Stadelmaier J, Eble J, Grummich K, Szczerba E, Kiesswetter E, et al. Substitution of animal-based with plant-based foods on cardiometabolic health and all-cause mortality: a systematic review and meta-analysis of prospective studies. BMC Med. 2023;21(1):404.
Chiavaroli L, Nishi SK, Khan TA, Braunstein CR, Glenn AJ, Mejia SB, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61(1):43–53.
Viguiliouk E, Kendall CW, Kahleova H, Rahelic D, Salas-Salvado J, Choo VL, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1133–45.
Glenn AJ, Guasch-Ferre M, Malik VS, Kendall CWC, Manson JE, Rimm EB, et al. Portfolio diet score and risk of cardiovascular disease: findings from 3 prospective cohort studies. Circulation. 2023;148(22):1750–63.
Glenn AJ, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, Kendall CWC, et al. Relationship between a plant-based dietary portfolio and risk of cardiovascular disease: findings from the Women’s Health Initiative prospective cohort study. J Am Heart Assoc. 2021;10(16): e021515.
Lo K, Glenn AJ, Yeung S, Kendall CWC, Sievenpiper JL, Jenkins DJA, Woo J. Prospective association of the portfolio diet with all-cause and cause-specific mortality risk in the Mr. OS and Ms. OS study. Nutrients. 2021;13(12):4360. https://doi.org/10.3390/nu13124360 .
Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, et al. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140(12):2302S-S2311.
Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):324. https://doi.org/10.3390/nu9040324 .
Download references
Acknowledgements
Aspects of this work were presented at the following conferences: Canadian Nutrition Society (CNS), Quebec City, Canada, May 4–6, 2023; 40th International Symposium on Diabetes and Nutrition, Pula, Croatia, June 15–18, 2023; and Nutrition 2023—American Society for Nutrition (ASN), Boston, USA, July 22–25, 2023.
Authors’ Twitter handles
@Toronto_3D_Unit.
This work was supported by the United Soybean Board (the United States Department of Agriculture Soybean Checkoff Program [funding reference number, 2411–108-0101]) and the Canadian Institutes of Health Research (funding reference number, 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3D) Centre, funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the conduct of this work. ME was funded by a CIHR Canada Graduate Scholarship and Toronto 3D PhD Scholarship award. DG was funded by an Ontario Graduate Scholarship. TAK and AZ were funded by a Toronto 3D Postdoctoral Fellowship Award. LC was funded by a Toronto 3D New Investigator Award. SA-C was funded by a CIHR Canadian Graduate Scholarship. DJAJ was funded by the Government of Canada through the Canada Research Chair Endowment. None of the sponsors had any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. But one of the co-authors, Mark Messina, who was involved in all aspects of the study except data collection or analysis, is the Director of Nutrition Science and Research at the Soy Nutrition Institute Global, an organization that receives partial funding from the principal funder, the United Soybean Board (USB).
Author information
Authors and affiliations.
Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
M. N. Erlich, D. Ghidanac, S. Blanco Mejia, T. A. Khan, L. Chiavaroli, A. Zurbau, S. Ayoub-Charette, L. A. Leiter, R. P. Bazinet, D. J. A. Jenkins, C. W. C. Kendall & J. L. Sievenpiper
Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition and Risk Factor Modification Centre, St. Michael’s Hospital, Toronto, ON, Canada
M. N. Erlich, D. Ghidanac, S. Blanco Mejia, T. A. Khan, L. Chiavaroli, A. Zurbau, S. Ayoub-Charette, L. A. Leiter, D. J. A. Jenkins, C. W. C. Kendall & J. L. Sievenpiper
Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON, Canada
L. Chiavaroli, L. A. Leiter, D. J. A. Jenkins & J. L. Sievenpiper
Royal College of Surgeons in Ireland, Dublin, Ireland
Soy Nutrition Institute Global, Washington, DC, USA
Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
L. A. Leiter, D. J. A. Jenkins & J. L. Sievenpiper
Division of Endocrinology and Metabolism, Department of Medicine, St. Michael’s Hospital, Toronto, ON, Canada
College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, SK, Canada
C. W. C. Kendall
You can also search for this author in PubMed Google Scholar
Contributions
The authors’ responsibilities were as follows: JLS designed the research (conception, development of overall research plan, and study oversight); ME and DG acquired the data; ME, SBM, TAK, and SAC performed the data analysis; JLS, ME, DG, SBM, AA, TAK, and LC interpreted the data; JLS and ME drafted the manuscript, have primary responsibility for the final content, and take responsibility for the integrity of the data and accuracy of the data analysis; JLS, MNE, DG, SBM, TAK, LC, AZ, SAC, AA, MM, LAL, RPB, CWCK, and DJD contributed to the project conception and critical revision of the manuscript for important intellectual content and read and approved the final version of the manuscript. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Corresponding author
Correspondence to J. L. Sievenpiper .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
TAK reports receiving grants from Institute for the Advancement of Food and Nutrition Sciences (IAFNS, formerly ILSI North America) and National Honey Board (USDA Checkoff program). He has received honorariums from Advancement of Food and Nutrition Sciences (IAFNS), the International Food Information Council (IFIC), the Calorie Control Council (CCC), the International Sweeteners Association (ISA), and AmCham Dubai. He has received funding from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. LC has received research support from the Canadian Institutes of health Research (CIHR), Protein Industries Canada (a Government of Canada Global Innovation Clusters), The United Soybean Board (USDA soy “Checkoff” program), and the Alberta Pulse Growers Association. AZ is a part-time research associate at INQUIS Clinical Research, Ltd., a contract research organization. She has received consulting fees from Glycemic Index Foundation Inc. SA-C has received an honorarium from the International Food Information Council (IFIC) for a talk on artificial sweeteners, the gut microbiome, and the risk for diabetes. MM was employed by the Soy Nutrition Institute Global, an organization that receives funding from the United Soybean Board (USB) and from members involved in the soy industry. RPB has received industrial grants, including those matched by the Canadian government, and/or travel support or consulting fees largely related to work on brain fatty acid metabolism or nutrition from Arctic Nutrition, Bunge Ltd., Dairy Farmers of Canada, DSM, Fonterra Inc, Mead Johnson, Natures Crops International, Nestec Inc. Pharmavite, Sancero Inc., and Spore Wellness Inc. Moreover, Dr. Bazinet is on the executive of the International Society for the Study of Fatty Acids and Lipids and held a meeting on behalf of Fatty Acids and Cell Signaling, both of which rely on corporate sponsorship. Dr. Bazinet has given expert testimony in relation to supplements and the brain. DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit Council (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI), and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from Lawson Centre Nutrition Digital Series, Nutritional Fundamentals for Health (NFH)-Nutramedica, Saint Barnabas Medical Center, The University of Chicago, 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978–0-12–810510-8), and his sister, Caroline Brydson, received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. He is also a vegan. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Lantmannen, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), National Honey Board (U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS), Pulse Canada, Quaker Oats Center of Excellence, INC International Nut and Dried Fruit Council Foundation, The United Soybean Board (USDA soy “Checkoff” program), Protein Industries Canada (a Government of Canada Global Innovation Cluster), Almond Board of California, European Fruit Juice Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF among other donors), The Plant Milk Fund at the University of Toronto (a fund established by the Karuna Foundation through Vegan Grants), and The Nutrition Trialists Network Fund at the University of Toronto (a fund established by donations from the Calorie Control Council and Physicians Committee for Responsible Medicine). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Danone, Nutrartis, Soylent, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from Danone, FoodMinds LLC, Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Arab Beverages, International Sweeteners Association, Association Calorie Control Council, and Phynova. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Ingredion, and Brightseed. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves as an unpaid member of the Board of Trustees of IAFNS. He is a Director at Large of the Canadian Nutrition Society (CNS), founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. All other authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3524_moesm1_esm.docx.
Additional file 1: This file contains Additional file 1 material, including the PRISMA checklist, further details on the search process, and additional results.
Flow of literature on the effect of substituting soymilk for cow’s milk on intermediate cardiometabolic outcomes. Exclusion criteria: duplicate, abstract only (conference abstract), non-human (animal study), in vitro, review/position paper/commentary/letter, observational (observational study), no soymilk (intervention was not soymilk), children (participants < 18 years of age), no suitable comparator (comparator was not cow’s milk), isolated soy protein (an ISP powder was given to participants), acute (follow-up of < 3 weeks), combined intervention (effects of intervention and comparator could not be isolated), wrong endpoint (no data for outcomes of interest), alternative publication (repeated data from original publication)
A summary plot for the effect of substituting soymilk for cow’s milk on intermediate cardiometabolic outcomes. Analyses were conducted using generic, inverse variance random-effects models (at least 5 trials available), or fixed-effects models (fewer than 5 trials available). Between-study heterogeneity was assessed by the Cochrane Q statistic, where P Q < 0.100 was considered statistically significant, and quantified by the I 2 statistic, where I 2 ≥ 50% was considered evidence of substantial heterogeneity. The GRADE of randomized controlled trials are rated as “high” certainty of evidence and can be downgraded by 5 domains and upgraded by 1 domain. The white squares represent no downgrades, the filled black squares indicate a single downgrade or upgrades for each outcome, and the black square with a white “2” indicates a double downgrade for each outcome. Because all included trials were randomized or nonrandomized controlled trials, the certainty of the evidence was graded as high for all outcomes by default and then downgraded or upgraded based on prespecified criteria. Criteria for downgrades included risk of bias (downgraded if most trials were considered to be at high ROB); inconsistency (downgraded if there was substantial unexplained heterogeneity: I 2 ≥ 50%; P Q < 0.10); indirectness (downgraded if there were factors absent or present relating to the participants, interventions, or outcomes that limited the generalizability of the results); imprecision (downgraded if the 95% CI crossed the minimally important difference (MID) for harm or benefit); and publication bias (downgraded if there was evidence of publication bias based on the funnel plot asymmetry and/or significant Egger or Begg test ( P < 0.10)), with confirmation by adjustment using the trim-and-fill analysis of Duval and Tweedie. The criteria for upgrades included a significant dose–response gradient. For the interpretation of the magnitude, we used the MIDs to assess the importance of magnitude of our point estimate using the effect size categories according to the new GRADE guidance. Then, we used the MIDs to assess the importance of the magnitude of our point estimates using the effect size categories according to the GRADE guidance as follows: a large effect (≥ 5 × MID); moderate effect (≥ 2 × MID); small important effect (≥ 1 × MID); and trivial/unimportant effect (< 1 MID). *HDL-C values reversed to show benefit. **LDL-C was not downgraded for imprecision, as the degree to which the upper 95% CI crosses the MID is not clinically meaningful. Additionally, the moderate change in non-HDL-C, with high certainty of evidence, substantiates the high certainty of the LDL-C results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Erlich, M.N., Ghidanac, D., Blanco Mejia, S. et al. A systematic review and meta-analysis of randomized trials of substituting soymilk for cow’s milk and intermediate cardiometabolic outcomes: understanding the impact of dairy alternatives in the transition to plant-based diets on cardiometabolic health. BMC Med 22 , 336 (2024). https://doi.org/10.1186/s12916-024-03524-7
Download citation
Received : 20 December 2023
Accepted : 09 July 2024
Published : 22 August 2024
DOI : https://doi.org/10.1186/s12916-024-03524-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Soy protein
- Cardiovascular disease
- Systematic review
- Meta-analysis
- Randomized controlled feeding trials
BMC Medicine
ISSN: 1741-7015
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Lifestyle medicine case manager nurses for type two diabetes patients: an overview of a job description framework—a narrative review.

1. Introduction
2. materials and methods, 2.1. study design, 2.2. identification of the research question, 2.3. inclusion and exclusion criteria, 2.4. search strategy, 2.5. data extraction and synthesis, 3.1. preliminary literature analysis, 3.2. literature screening, 3.3. general characteristics of studies included, 3.4. overview of the role and clinical applications of lmcmns at the international level, 3.5. overview of the role and clinical applications of specialist nurses in italy, 3.6. academic pathways for specialist nurses and case managers in italy, 3.7. job description for delphi method purposes, 4. discussion, limitations, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest, appendix a. search strategy, appendix a.1. pubmed search strategy, appendix a.2. scopus search strategy.
- Hivert, M.F.; Arena, R.; Forman, D.E.; Kris-Etherton, P.M.; McBride, P.E.; Pate, R.R.; Spring, B.; Trilk, J.; Van Horn, L.V.; Kraus, W.E. Medical Training to Achieve Competency in Lifestyle Counseling: An Essential Foundation for Prevention and Treatment of Cardiovascular Diseases and Other Chronic Medical Conditions: A Scientific Statement From the American Heart Association. Circulation 2016 , 134 , e308–e327. [ Google Scholar ] [ CrossRef ]
- Sagner, M.; Kats, D.; Egger, G.; Lianov, L.; Schulz, K.H.; Braman, M.; Behbod, B.; Phillips, E.; Dysinger, W.; Ornish, D. Lifestyle medicine potential for reversing a world of chronic disease epidemics: From cell to community. Int. J. Clin. Pract. 2014 , 68 , 1289–1292. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Minich, D.M.; Bland, J.S. Personalized lifestyle medicine: Relevance for nutrition and lifestyle recommendations. Sci. World J. 2013 , 2013 , 129841. [ Google Scholar ] [ CrossRef ]
- Bodai, B.I.; Tuso, P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 2015 , 19 , 48–79. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hyman, M.A.; Ornish, D.; Roizen, M. Lifestyle medicine: Treating the causes of diseases. Altern. Ther. Health Med. 2009 , 15 , 12–14. [ Google Scholar ] [ PubMed ]
- Ford, E.S.; Bergmann, M.M.; Kröger, J.; Schienkiewitz, A.; Weikert, C.; Boeing, H. Healthy living is the best revenge: Findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Arch. Intern. Med. 2009 , 169 , 1355–1362. [ Google Scholar ] [ CrossRef ]
- International Diabetes Federation, IDF. IDF Guide for Diabetes Epidemiology Studies. Available online: http://www.addthis.com/bookmark.php (accessed on 1 January 2024).
- Istituto Superiore di Sanità, ISS. Giornata Mondiale Diabete: Dalla Prevalenza ALL’ACCESSO Alle Cure, i Numeri della Sorveglianza Passi. Available online: https://www.iss.it/-/giornata-mondiale-diabete-da-prevalenza-ad-accesso-cure-i-numeri-del-sistema-passi (accessed on 3 July 2024).
- Istituto Nazionale di Statistica. Available online: https://www.istat.it/it/archivio/202600 (accessed on 1 January 2024).
- American Diabetes Association. Standards of Medical Care in Diabetes 2017 Abridged for Primary Care Providers. Diabetes Care. 2017 , 40 (Suppl. 1), S1–S135. [ Google Scholar ] [ CrossRef ]
- American Case Management Association, ACMA. Standards of Practice & Scope of Services. 2020. Available online: www.acmaweb.org/Standards (accessed on 1 January 2024).
- O’Flynn, S. Nurses’ role in diabetes management and prevention in community care. Br. J. Community Nurs. 2022 , 27 , 374–376. [ Google Scholar ] [ CrossRef ]
- Ahmed, S.K. The Impact of COVID-19 on Nursing Practice: Lessons Learned and Future Trends. Cureus 2023 , 15 , e50098. [ Google Scholar ] [ CrossRef ]
- Joo, J.Y.; Huber, D.L. An Integrative Review of Case Management for Diabetes. Prof. Case Manag. 2012 , 17 , 72–85. [ Google Scholar ] [ CrossRef ]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022 , 14 , 414–417. [ Google Scholar ] [ CrossRef ]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club. 1995 , 123 , A12–A13. [ Google Scholar ] [ CrossRef ]
- Cangelosi, G.; Grappasonni, I.; Pantanetti, P.; Scuri, S.; Garda, G.; Nguyen, C.C.T.; Petrelli, F. Nurse Case Manager Lifestyle Medicine (NCMLM) in the Type Two Diabetes patient concerning post COVID-19 Pandemic management: Integrated-Scoping literature review. Ann. Ig. 2022 , 34 , 585–602. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ministero della Salute. Piano Nazionale della Prevenzione 2020–2025. Direzione Generale Prevenzione. Accordo Tra lo Stato, le Regioni e le Province Autonome di Trento e di Bolzano. 6 August 2020. Available online: https://www.salute.gov.it/imgs/C_17_notizie_5029_0_file.pdf (accessed on 19 April 2024).
- Governo Italiano. Presidenza del Consiglio dei Ministri. “Piano Nazionale di Ripresa e Resilienza”. Available online: https://www.governo.it/sites/governo.it/files/PNRR.pdf (accessed on 19 April 2022).
- Governo Italiano. Presidenza del Consiglio dei Ministri. Decreto Ministeriale 71, “Modelli e Standard per lo Sviluppo dell’Assistenza Territoriale nel Servizio Sanitario Nazionale (Consiglio dei Ministri del 21.04.22)”. Available online: https://www.gazzettaufficiale.it/eli/id/2022/05/03/22A02656/sg (accessed on 1 June 2024).
- Governo Italiano. Presidenza del Consiglio dei Ministri. Decreto-Legge del 19/05/2020 n. 34. “Misure Urgenti in Materia di Salute, Sostegno al Lavoro e All’Economia, Nonché di Politiche Sociali Connesse All’Emergenza Epidemiologica da COVID-19”. Available online: https://www.gazzettaufficiale.it/eli/id/2020/05/19/20G00052/sg (accessed on 1 June 2024).
- Agenzia Nazionale per i Servizi Sanitari Regionali, Agenas. Linee di Indirizzo Infermiere di Famiglia e Comunità. 2023. Available online: https://www.agenas.gov.it/comunicazione/primo-piano/2298-agenas-pubblica-le-linee-di-indirizzo-infermiere-di-famiglia-o-comunit%C3%A0 (accessed on 10 June 2024).
- Shaban, M.M.; Sharaa, H.M.; Amer, F.G.M.; Shaban, M. Effect of digital based nursing intervention on knowledge of self-care behaviors and self-efficacy of adult clients with diabetes. BMC Nurs. 2024 , 23 , 130. [ Google Scholar ] [ CrossRef ]
- Yaagoob, E.; Lee, R.; Stubbs, M.; Shuaib, F.; Johar, R.; Chan, S. WhatsApp-based intervention for people with type 2 diabetes: A randomized controlled trial. Nurs. Health Sci. 2024 , 26 , e13117. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Park, S.; Park, J.E. Effects of digital self-care intervention for Korean older adults with type 2 diabetes: A randomized controlled trial over 12 weeks. Geriatr. Nurs. 2024 , 58 , 155–161. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tamiru, S.; Dugassa, M.; Amsalu, B.; Bidira, K.; Bacha, L.; Tsegaye, D. Effects of Nurse-Led diabetes Self-Management education on Self-Care knowledge and Self-Care behavior among adult patients with type 2 diabetes mellitus attending diabetes follow up clinic: A Quasi-Experimental study design. Int. J. Afr. Nurs. Sci. 2023 , 18 , 100548. [ Google Scholar ] [ CrossRef ]
- World Health Organization, WHO. One Health. Available online: https://www.who.int/health-topics/one-health#tab=tab_1 (accessed on 15 June 2024).
- Ministero della Salute Italiano. Il Processo di Health Technology Assessment (HTA). Available online: https://www.salute.gov.it/portale/dispositiviMedici/dettaglioContenutiDispositiviMedici.jsp?lingua=italiano&id=5199&area=dispositivi-medici&menu=tecnologie (accessed on 5 April 2024).
- World Health Organization, WHO. 2015 Global Survey on Health Technology Assessment by National Authorities. Available online: https://www.who.int/publications/i/item/9789241509749 (accessed on 1 May 2024).
- Federazione Nazionale Ordini delle Professioni Infermieristiche, FNOPI. Evoluzione delle Competenze Infermieristiche. Delibera n. 79 del 25 Aprile 2015. Available online: https://www.fnopi.it/ (accessed on 15 June 2024).
- Oberle, K.; Kathleen, M.; Allen, M. The nature of advanced practice nursing. Nurs. Outlook 2001 , 49 , 148–153. [ Google Scholar ] [ CrossRef ]
- American Nurses Association, ANA. Clinical Case Management Practice. In Nursing Case Management Review and Resource Manual , 4th ed.; American Nurses Association, ANA: Annapolis, MD, USA, 2012; Available online: https://www.nursingworld.org/ (accessed on 15 June 2024).
- Parlamento della Repubblica Italiana. Legge 26 Febbraio 1999, n. 42. Disposizioni in Materia di Professioni Sanitarie. Available online: https://www.gazzettaufficiale.it/eli/id/1999/03/02/099G0092/sg (accessed on 1 May 2024).
- Ministero della Sanità Italiano. Decreto 14 Settembre 1994, n. 739. Regolamento Concernente L’Individuazione della Figura e del Relativo Profilo Professionale Dell’Infermiere. Available online: https://www.gazzettaufficiale.it/eli/id/1995/01/09/095G0001/sg (accessed on 5 May 2024).
- Ministro Dell’Istruzione, Dell’Università e della Ricerca di Concerto con Il Ministro del Lavoro, della Salute e delle Politiche Sociali Italiano. Decreto Interministeriale 19 Febbraio 2009. Determinazione delle Classi delle Lauree delle Professioni Sanitarie, ai Sensi del Decreto Ministeriale 22 Ottobre 2004, n. 270. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2009-05-25&atto.codiceRedazionale=09A05797&elenco30giorni=false (accessed on 1 April 2024).
- Parlamento della Repubblica Italiana. Legge 1 Febbraio 2006, n. 43. Disposizioni in Materia di Professioni Sanitarie Infermieristiche, Ostetrica, Riabilitative, Tecnico-Sanitarie e della Prevenzione e Delega al Governo per L’Istituzione dei Relativi Ordini Professionali. Available online: https://www.gazzettaufficiale.it/eli/id/2006/02/17/006G0050/sg (accessed on 5 April 2024).
- Presidenza della Repubblica Italiana. Decreto del Presidente della Repubblica 13 Giugno 2023, n. 81. Regolamento Concernente Modifiche al Decreto del Presidente della Repubblica 16 Aprile 2013, n. 62, Recante: «Codice di Comportamento dei Dipendenti Pubblici, a Norma Dell’Articolo 54 del Decreto Legislativo 30 Marzo 2001, n. 165». Available online: https://www.gazzettaufficiale.it/eli/id/2023/06/29/23G00092/sg (accessed on 5 April 2024).
- Parlamento della Repubblica Italiana. Legge 8 marzo 2017, n. 24. Disposizioni in Materia di Sicurezza delle Cure e della Persona Assistita, Nonchè in Materia di Responsabilità Professionale Degli Esercenti le Professioni Sanitarie. Available online: https://www.gazzettaufficiale.it/eli/id/2017/03/17/17G00041/sg (accessed on 15 April 2024).
- Parlamento della Repubblica Italiana. Legge 11 Gennaio 2018, n. 3. Delega al Governo in Materia di Sperimentazione Clinica di Medicinali Nonche’ Disposizioni per Il Riordino delle Professioni Sanitarie e per la Dirigenza Sanitaria del Ministero della Salute. Available online: https://www.gazzettaufficiale.it/eli/id/2018/1/31/18G00019/sg (accessed on 15 April 2024).
- Federazione Nazionale Ordini delle Professioni Infermieristiche, FNOPI. Codice Deontologico delle Professioni Infermieristiche. 2019. Available online: https://www.fnopi.it/ (accessed on 15 June 2024).
- Agenzia per la Rappresentanza Negoziale delle Pubbliche Amministrazioni, ARAN. Contratto Collettivo Nazionale Lavoratori (CCNL) Comparto Sanità Triennio 2016–2018 il Siglato il 21 Maggio 2018. Available online: https://www.asp.cz.it/files/Relazioni%20Sindacali/CCNL%20comparto%20sanit%C3%A0%20triennio%202016-2018%20-%20firmato%20da%20NURSING%20UP%20e%20CSE.pdf (accessed on 15 June 2024).
- Parlamento della Repubblica Italiana. Legge 24 Dicembre 2007, n. 244. Disposizioni per la Formazione del Bilancio Annuale e Pluriennale dello Stato (Legge Finanziaria 2008). Available online: https://www.gazzettaufficiale.it/eli/id/2007/12/28/007G0264/sg (accessed on 15 June 2024).
- van der Feltz-Cornelis, C.; Attree, E.; Heightman, M.; Gabbay, M.; Allsopp, G. Integrated Care pathways: A new approach for integrated care systems. Br. J. Gen. Pract. 2023 , 73 , 422. [ Google Scholar ] [ CrossRef ]
- Reig-Garcia, G.; Cámara-Liebana, D.; Suñer-Soler, R.; Pau-Perich, E.; Sitjar-Suñer, M.; Mantas-Jiménez, S.; Roqueta-Vall-Llosera, M.; Malagón-Aguilera, M.d.C. Assessmentof Standardized Care Plans for People with Chronic Diseases in Primary Care Settings. Nurs. Rep. 2024 , 14 , 801–815. [ Google Scholar ] [ CrossRef ]
- Ghiyasvandian, S.; Shahsavari, H.; Matourypour, P.; Golestannejad, M.R. Integrated Care model: Transition from acute to chronic care. Rev. Bras. Enferm. 2021 , 74 (Suppl. 5), e20200910. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abraham, C.M.; Norful, A.A.; Stone, P.W.; Poghosyan, L. Cost-Effectiveness of Advanced Practice Nurses Compared to Physician-Led Care for Chronic Diseases: A Systematic Review. Nurs. Econ. 2019 , 37 , 293–305. [ Google Scholar ] [ PubMed ]
- Woo, B.F.Y.; Lee, J.X.Y.; Tam, W.W.S. The impact of the advanced practice nursing role on quality of care, clinical outcomes, patient satisfaction, and cost in the emergency and critical care settings: A systematic review. Hum. Resour. Health 2017 , 15 , 63. [ Google Scholar ] [ CrossRef ]
- Martin, C.M.; Peterson, C.; Robinson, R.; Sturmberg, J.P. Care for chronic illness in Australian general practice-focus groups of chronic disease self-help groups over 10 years: Implications for chronic care systems reforms. Asia Pac. Fam. Med. 2009 , 8 , 1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- National Health Service (NHS), Lakeside Healthcare Group. Chronic Disease Specialist Nurse. 2024. Available online: https://www.jobs.nhs.uk/candidate/jobadvert/A3007-23-0223?sort=closingDate&language=&page=861#:~:text=As%20a%20Specialist%20Nurse%2C%20you,patient%20and%20other%20health%20professionals (accessed on 3 August 2024).
- Petrelli, F.; Cangelosi, G.; Nittari, G.; Pantanetti, P.; Debernardi, G.; Scuri, S.; Sagaro, G.G.; Nguyen, C.T.T.; Grappasonni, I. Chronic Care Model in Italy: A narrative review of the literature. Prim. Health Care Res. Dev. 2021 , 22 , e32. [ Google Scholar ] [ CrossRef ]
- Grudniewicz, A.; Gray, C.S.; Boeckxstaens, P.; De Maeseneer, J.; Mold, J. Operationalizing the Chronic Care Model with Goal-Oriented Care. Patient 2023 , 16 , 569–578. [ Google Scholar ] [ CrossRef ]
- Stellefson, M.; Dipnarine, K.; Stopka, C. The chronic care model and diabetes management in US primary care settings: A systematic review. Prev. Chronic Dis. 2013 , 10 , E26. [ Google Scholar ] [ CrossRef ]
- Nasa, P.; Jain, R.; Juneja, D. Delphi methodology in healthcare research: How to decide its appropriateness. World J. Methodol. 2021 , 11 , 116–129. [ Google Scholar ] [ CrossRef ]
- Giusti, E.M.; Veronesi, G.; Callegari, C.; Borchini, R.; Castelnuovo, G.; Gianfagna, F.; Iacoviello, L.; Ferrario, M.M. Pre-pandemic burnout and its changes during the COVID-19 outbreak as predictors of mental health of healthcare workers: A lesson to be learned. Psychiatry Res. 2023 , 326 , 115305. [ Google Scholar ] [ CrossRef ]
- Palese, A.; Chiappinotto, S.; Fonda, F.; Visintini, E.; Peghin, M.; Colizzi, M.; Balestrieri, M.; De Martino, M.; Isola, M.; Tascini, C. Lessons learnt while designing and conducting a longitudinal study from the first Italian COVID-19 pandemic wave up to 3 years. Health Res. Policy Syst. 2023 , 21 , 111. [ Google Scholar ] [ CrossRef ]
- Willems, S.; Vanden Bussche, P.; Van Poel, E.; Collins, C.; Klemenc-Ketis, Z. Moving forward after the COVID-19 pandemic: Lessons learned in primary care from the multi-country PRICOV-19 study. Eur. J. Gen. Pract. 2024 , 30 , 2328716. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marcadelli, S.; Stievano, A.; Rocco, G. Policy proposals for a new welfare: The development of the family and community nurse in Italy as the key to promote social capital and social innovation. Prim. Health Care Res. Dev. 2019 , 20 , e109. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Federazione Nazionale Ordini Professioni Infermieristiche, FNOPI. Position Statement L’Infermiere di Famiglia e di Comunità. 2020. Available online: https://www.fnopi.it/en/ (accessed on 22 May 2024).
- Bagnasco, A.; Catania, G.; Zanini, M.; Pozzi, F.; Aleo, G.; Watson, R.; Hayter, M.; Sasso, L.; Rodrigues, C.; Alvino, S.; et al. Core competencies for family and community nurses: A European e-Delphi study. Nurse Educ. Pract. 2022 , 60 , 103296. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Konswa, A.A.; Alolaiwi, L.; Alsakkak, M.; Aleissa, M.; Alotaibi, A.; Alanazi, F.F.; Bin Rasheed, A. Experience of establishing a lifestyle medicine clinic at primary care level- challenges and lessons learnt. J. Taibah Univ. Med. Sci. 2023 , 18 , 1364–1372. [ Google Scholar ] [ CrossRef ]
- Fernández Guijarro, S.; Pomarol-Clotet, E.; Rubio Muñoz, M.C.; Miguel García, C.; López, E.E.; Guijarro, R.F.; Pérez, L.C.; Cuadra, M.A.R. Effectivenessof a community-based nurse-led lifestyle-modification intervention for people with serious mental illness and metabolic syndrome. Int. J. Ment. Health Nurs. 2019 , 28 , 1328–1337. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Author/Year | Country | Study Design | Timing | Sample | Principal Interventions | Results |
|---|---|---|---|---|---|---|
| Shaban MM et al., 2024 [ ] | Egypt | Quasi-experimental | 6 months | Experimental group (n = 60) Control group (n = 60) | Digital-based nursing intervention for diabetes education and lifestyle behavior | The intervention group demonstrated improvements in diet, exercise, medication adherence, blood glucose testing, and foot care |
| Yaagoob E et al., 2024 [ ] | Saudi Arabia | RCT | 6- and 12-week follow-up | Experimental group (n = 40) Control group (n = 40) | Use of social media for diabetes education and lifestyle behavior | Significant increase in self-efficacy, self-management, and education in the experimental group |
| Park S et al., 2024 [ ] | South Corea | RCT | 12 weeks | Intervention group (n = 60) Control group (n = 60) | Specific App used for diabetes education and lifestyle behavior | The digital self-care intervention was beneficial for blood sugar control |
| Tamiru S et al., 2023 [ ] | Ethiopia | Quasi-experimental | 5 months | Intervention group (n = 180) Control group (n = 180) | Nurse-led diabetes self-management education (DSME)-structured | Substantial improvement in diabetes knowledge in the experimental group |
| JD FOR LMCMN IN DIABETOLOGY | |
|---|---|
| Regulatory and Legal Framework | |
| Qualification: | Nurse—Category D. |
| Minimum Education Requirement | Bachelor’s degree in Nursing Science or equivalent titles as per Law 42/1999 “Provisions on healthcare professions” [ ]. |
| Career Titles | -PhD; -Master’s degree in nursing and midwifery (LM/SNT1); -First- or second-level Master’s degree in diabetes and/or metabolic diseases; -First- or second-level Master’s degree in LM. |
| Institutional Obligations | Registration with the National Federation of Nursing Professions. |
| Key Regulatory, Legislative, and Ethical References | -DM 739/1994, “Regulation regarding the identification and professional profile of the Nurse” [ ]; -Law 42/1999, “Provisions on healthcare professions” [ ]; -MIUR Interministerial Decree of 19 February 2009, “Determination of degree classes for healthcare professions” [ ]; -Law 43/2006, “Provisions on nursing, midwifery, rehabilitation, technical-health, and prevention professions, and delegation to the Government for the establishment of related professional orders” [ ]; -DPR 62 of 16 April 2013, “Regulation containing the Code of Conduct for public employees, pursuant to Article 54 of Legislative Decree 30 March 2001, No. 165” [ ]; -Law 24/2017, “Provisions on the safety of care and the assisted person, as well as on the professional liability of healthcare professionals” [ ]; -Law 3/2018, “Delegation to the Government on clinical trials of medicinal products, and provisions for the reorganization of health professions and for the management of the Ministry of Health” [ ]; -2019 Code of Ethics for Nursing Professions [ ]. |
| Contractual References | National Collective Labor Agreement (CCNL) for the Healthcare Sector 2016–2018, signed on 21 May 2018 [ ]. |
| Training | Participates in company and departmental training programs and, in accordance with Article 2, paragraph 357 of Law 244/2007 of 24 December 2007 [ ] and subsequent amendments and integrations, complies with the guidelines for Continuing Medical Education (CME). Enhances personal cultural knowledge by supporting and assisting in clinical, care, and social health activities alongside nursing students during their training internships. |
| Research | Engages in research and continuous improvement activities. Based on the competencies of their profile and the observation of their professional activity, promotes research projects and the development of specific skills typical of the nursing profession from an LM perspective. |
| Information Flow | Participates in all health management activities, utilizing the necessary tools to observe performance and socio-health phenomena. Specifically, updates the electronic medical record used at the center on a daily basis. |
| Responsibilities | The LM nurse specializing in diabetology is responsible for providing nursing care to patients with diabetes and endocrine disorders. Care for individuals, the community, and families is delivered through specific autonomous and multidisciplinary interventions in the areas of prevention, promotion, and rehabilitation of therapeutic treatments within an LM framework. By integrating with the multidisciplinary team, the nurse implements the nursing process in the phases of Assessment, Diagnosis, Planning, Implementation, and Evaluation of the Individualized Care Plan (ICP) for patients with diabetes and/or endocrine disorders. |
| Objectives | Ensure that the nursing needs of patients with diabetes are met, providing consistent care throughout all phases of the ICP. |
| Direct Reporting Line | Reports directly to the Responsible Manager and the relevant Organizational Function. |
| Indirect Reporting Line | Reports indirectly to the Director of Nursing and Midwifery Services and the relevant Organizational Position. |
| Cross-functionality | In a multidimensional/multidisciplinary approach, collaborates with all healthcare professionals assisting patients in an outpatient setting at the center. |
| Third-Sector Engagement | Promotes and interacts with all patient and family associations that work in synergy with the reference center. |
| Space and Time Management | Organizes spaces and reception modalities for individuals with metabolic and/or endocrine disorders, coordinating with the team to ensure that all clinical, care, and social health activities are conducted according to LM principles. |
| Tools | Utilizes all available tools to promote multidisciplinary and interdepartmental integration (shared medical record and/or electronic supports). |
| Major Interventions During Nursing Assessment | -Arrange spaces and environments to provide the best reception for the patient, their family, and their community from an LM perspective. -Observe signs and symptoms expressed by the patient or their family, identifying LM needs. -Encourage the patient, family, or community to voice their concerns and seek help. -Collect anamnesis and clinical data, assessing the care priorities for the patient, family, or community. -Measure vital signs and identify the patient’s needs from an LM perspective. -Assess the resources available to the patient, family, and community in terms of autonomy to meet LM needs. -Identify the primary caregiver to be involved in the ICP process. |
| Major Interventions During Nursing Diagnosis and Care Objectives | Analyze the collected data to develop LM nursing diagnoses that address the care needs of patients with diabetes and/or endocrine disorders, as well as their families and communities. Collaborate and integrate with the multidisciplinary team to assess clinical care and social healthcare priorities from an LM perspective. |
| Major Interventions During Nursing Planning | -Collaborate with the multidisciplinary care team to develop the ICP from an LM perspective. -Facilitate the development of pathways and procedures for continuous LM care in a multidisciplinary approach. -Promote and support the development of specific LM professional standards. -Plan LM therapeutic or diagnostic interventions. |
| Major Interventions for Nursing Implementation | -Implement the ICP from an LM perspective. -Support the relationship with the patient, their family, and their community through a listening-centered approach, focusing on patients with diabetes and/or endocrine disorders. -Guide and support the patient, their family, and their community through all phases of the ICP. -Perform necessary LM nursing practices for the care and rehabilitation of patients with diabetes and/or endocrine disorders, their families, and their communities, working interdependently. -Foster the development of a supportive network to achieve care objectives. -Interact with the family and community throughout the ICP process. -Implement nursing interventions, defining the necessary time, methods, tools, and material and immaterial resources. -Properly manage clinical care documentation in all its parts and within appropriate timeframes. -Apply company procedures, protocols, and departmental operational instructions. -Review and update the ICP based on the responses of patients with diabetes and/or endocrine disorders, their families, and their communities. -Integrate new care tools, such as technological devices and new communication forms (tele-nursing LM). |
| Major Interventions During Nursing Evaluation | -Evaluate the ICP as a whole, suggesting possible LM improvement strategies. -Monitor the interventions provided, verifying both direct and indirect outcomes of the care given. -Document the outcomes of interventions using appropriate departmental and corporate communication and information tools. -Suggest possible improvement strategies by evaluating and comparing the planned and actual timelines of the entire LM nursing process for patients with diabetes and/or endocrine disorders, their families, and their communities. |
| Major Interventions in Therapeutic Education and Health Prevention | -Develop LM educational–therapeutic programs to promote healthy and conscious lifestyles for patients with eating disorders, as well as for their families and communities. -Identify educational and preventive health needs for patients with diabetes and/or endocrine disorders, as well as for their families and communities. -Identify major risk factors for patients with diabetes and/or endocrine disorders and facilitate the development and implementation of specific primary, secondary, and tertiary prevention programs within the LM framework. -Promote the production of LM informational materials for both individualized educational–therapeutic purposes and community-wide prevention. -Promote individualized nursing care plans according to the principles of LM. -Provide specialist LM nursing consultancy as needed. |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Cangelosi, G.; Mancin, S.; Pantanetti, P.; Nguyen, C.T.T.; Morales Palomares, S.; Biondini, F.; Sguanci, M.; Petrelli, F. Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review. Diabetology 2024 , 5 , 375-388. https://doi.org/10.3390/diabetology5040029
Cangelosi G, Mancin S, Pantanetti P, Nguyen CTT, Morales Palomares S, Biondini F, Sguanci M, Petrelli F. Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review. Diabetology . 2024; 5(4):375-388. https://doi.org/10.3390/diabetology5040029
Cangelosi, Giovanni, Stefano Mancin, Paola Pantanetti, Cuc Thi Thu Nguyen, Sara Morales Palomares, Federico Biondini, Marco Sguanci, and Fabio Petrelli. 2024. "Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review" Diabetology 5, no. 4: 375-388. https://doi.org/10.3390/diabetology5040029
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 20 August 2024
Orbital floor fracture (blow out) and its repercussions on eye movement: a systematic review
- Ilan Hudson Gomes de Santana 1 ,
- Mayara Rebeca Martins Viana 2 ,
- Julliana Cariry Palhano-Dias 3 ,
- Osny Ferreira-Júnior 4 ,
- Eduardo Sant’Ana 4 ,
- Élio Hitoshi Shinohara 5 &
- Eduardo Dias Ribeiro 6
European Journal of Medical Research volume 29 , Article number: 427 ( 2024 ) Cite this article
64 Accesses
Metrics details
The aim of this systematic review was to investigate the relationship between fractures of the floor of the orbit (blow outs) and their repercussions on eye movement, based on the available scientific literature. In order to obtain more reliable results, we opted for a methodology that could answer the guiding question of this research. To this end, a systematic review of the literature was carried out, using a rigorous methodological approach. The risk of bias was assessed using version 2 of the Cochrane tool for the risk of bias in randomized trials (RoB 2). This systematic review was carried out according to a systematic review protocol previously registered on the PROSPERO platform. The searches were carried out in the PubMed (National Library of Medicine), Scopus, ScienceDirect, SciELO, Web of Science, Cochrane Library and Embase databases, initially resulting in 553 studies. After removing duplicates, 515 articles remained, 7 were considered eligible, of which 3 were selected for detailed analysis. However, the results of the included studies did not provide conclusive evidence of a direct relationship between orbital floor fractures and eye movement.
Introduction
The anatomy of the orbit is a complex and vital structure, made up of seven distinct bones that define its boundaries [ 1 ]. Within this pyramid-shaped bone cavity, a variety of essential elements are present, including the eyeball, fat, extraocular muscles, nerves, blood vessels, lacrimal sac and lacrimal gland [ 2 ]. Its lateral and medial walls are outlined by a combination of bones, most notably the greater wing of the sphenoid bone and the zygomatic bone in the lateral wall, and the lacrimal bone, ethmoid bone, maxilla and lesser wing of the sphenoid in the medial wall [ 3 , 4 ]. The orbital floor, formed mainly by the maxilla and the zygomatic bone, plays a fundamental role in maintaining the normal structure and function of the orbit. Its delicate curvature, which extends smoothly from the inferior orbital rim to the superior orbital fissure, is important in preventing complications such as enophthalmos in cases of orbital fractures [ 5 , 6 ].
Orbital fractures are injuries to the bones surrounding the orbit and represent the third most common type of facial fracture in adults and children [ 7 , 8 ]. They are generally classified based on their anatomical location, including fractures of the orbital floor, orbital roof, lateral wall and medial wall [ 9 , 10 ]. Blunt trauma to the ocular region is the main mechanism of injury, often resulting in fracture of the thin bones of the orbit, especially the floor and medial wall [ 11 , 12 ]. These injuries occur due to the transmission of kinetic energy from the bones around the eye or due to increased pressure when the eyeball presses on the orbit. They are also known as blow-out fractures, as they tend to move away from the orbit [ 6 , 13 ].
Thus, the etiology of orbital floor fractures, as well as other types of maxillofacial trauma, includes traffic accidents, assaults, falls, sports injuries, firearm injuries and other incidents [ 14 ]. In addition, industrial accidents have also been identified as a source of trauma [ 15 ]. In developing countries, such as India, traffic accidents are one of the main causes of trauma, while in studies carried out elsewhere, assaults are often cited as the main cause. Worldwide, men are significantly more affected by maxillofacial trauma than women, accounting for approximately 85% of cases [ 16 , 17 ].
In addition, the diagnosis of these fractures is based on physical examination and imaging tests. On physical examination, signs and symptoms such as periorbital ecchymosis, limited eye movement, diplopia and enophthalmos may be present [ 18 ]. Computed tomography is the most efficient test for diagnosing these fractures. Treatment should be carried out by reconstructing the fractured orbital walls with autogenous, homogenous, heterogenous or alloplastic biomaterials [ 18 , 19 , 20 ].
Therefore, the aim of this systematic review was to determine, based on the available scientific literature, the relationship between the fracture of the floor of the orbit (known as blow out) and its consequences for eye movement.
Materials and methods
In order to obtain more reliable results, we opted for a methodology that could answer the guiding question of this research. To this end, a systematic review of the literature], to assess the relationship between orbital floor fracture (blow out) and the repercussions on the ocular. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was used to write the study [ 21 ]. The process followed criteria predefined by a Systematic Review Protocol registered with PROSPERO [ 22 ], guiding the selection and analysis of articles to provide a comprehensive overview of current knowledge on the subject. The methodological analysis included a clear protocol for selecting studies, extracting data and assessing methodological quality, maintaining transparency and rigor to guarantee the validity of the results. Strategies were adopted to evaluate and mitigate errors, including standardized training, initial testing, consensus meetings between evaluators and continuous monitoring. A double-blind review was carried out at all stages. When there was a small conflict regarding the exclusion of an article, a third independent reviewer was asked to resolve the disagreement, ensuring clear and consistent criteria. Once this conflict was resolved, the third reviewer excluded the paper, as did the first, as the study did not answer the research question.
Development and registration of the systematic review protocol
A meticulous protocol, covering all the essential elements of the methodology of a systematic review, was drawn up and submitted for approval on the PROSPERO (Prospective Register of Systematic Reviews) [ 22 ] platform prior to the start of this study. This protocol covered several aspects in detail, including defining the start and end date of the study, formulating the research question, the databases searched, structuring the acronym PICO (patient, intervention, comparison, outcome), designing a precise search strategy, stipulating inclusion and exclusion criteria for the study, determining outcome measures, screening methods, data extraction and analysis, as well as the approach to data synthesis. The prior registration of this protocol in the International Prospective Register of Systematic Reviews (PROSPERO) [ 22 ] was carried out in order to guarantee the transparency, integrity and methodological quality of this systematic review.
This systematic review was conducted in accordance with a systematic review protocol previously registered on the PROSPERO platform, identified by the number CRD42024497638.
PICO question
The use of the PICO components (Patient, Intervention, Comparison and Outcome) played a crucial role in defining the search strategy for evidence and the subsequent analysis of this systematic review. This specific approach was key to locating relevant studies and played a vital role in ensuring objectivity during the assessment of this work. Patient (P): Individuals diagnosed with an orbital floor fracture; Intervention (I): Exposure to orbital floor fracture; Comparison (C): Individuals without an orbital floor fracture; Outcome (O): The repercussions on eye movement, including changes in motility, diplopia and other related changes.
Guiding research question
The research question was formulated as follows: What is the relationship between orbital floor fracture (blow out) and repercussions on eye movement?
Search strategy and selection of articles
The electronic bibliographic searches were carried out through systematic searches in the PubMed (National Library of Medicine), Scopus, ScienceDirect, SciELO, Web of Science, Cochrane Library and Embase databases. Search terms and Boolean operators (AND and OR) were combined to better perform the searches in the databases, and the following search strategy was formulated:: Fractures AND Ocular Motility Disorders) OR (Oculomotor Nerve Injuries AND Ocular Motility AND Orbital Fractures AND Facial Trauma) OR (Ocular Trauma OR Orbital Fractures AND Ocular Motility AND Muscle Damage) Portuguese strategy: (Relation AND Orbital Fractures AND Ocular Motility Disorders) OR (Oculomotor Nerve Injuries AND Ocular Motility AND Orbital Fractures AND facial trauma) OR (Ocular trauma AND Orbital Fractures AND Ocular Motility AND muscle damage).
The methodological quality of the articles chosen was assessed by two independent researchers, considering both the title and the abstract (when available). The aim was to check whether these articles met the inclusion criteria; when the information in the abstract was insufficient to determine the inclusion of the study, the full text was read. After individual assessments, the researchers reached a consensus on the inclusion of studies for full text analysis.
Criteria for selection, inclusion and exclusion of studies
We included studies in English, Spanish, Japanese, Chinese, German and Portuguese which were randomized clinical trials, systematic reviews, cohort studies, case–control studies, cross-sectional studies, detailed case reports, with a sample made up of patients of all ages and both sexes who had suffered a fracture of the floor of the orbit. All studies that did not meet the inclusion criteria for this study, such as patients with medical conditions that could significantly interfere with the association between orbital floor fracture and eye movement, were excluded.
Selection of studies
The database search resulted in the initial identification of 553 studies. After removing duplicates using Rayyan © software [ 23 ], 515 articles remained, as shown in Fig. 1 . Of these, 7 were considered eligible according to the inclusion criteria and were selected for a more detailed analysis. After a thorough evaluation, taking into account the inclusion and exclusion criteria, 3 studies were identified as particularly relevant and included in this systematic review.
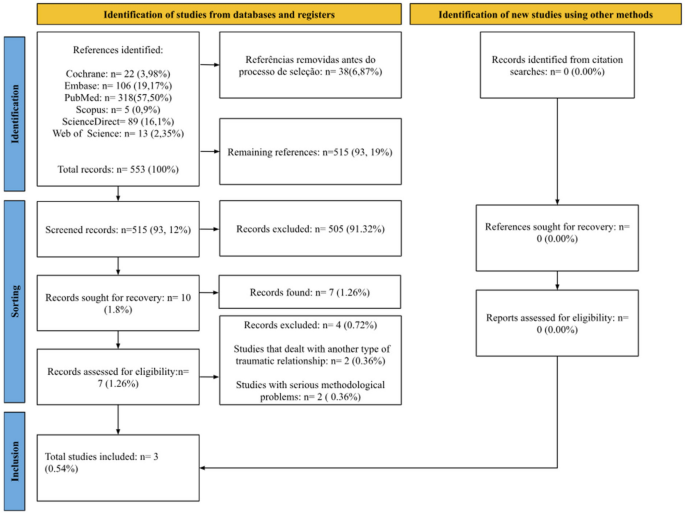
Source: authors (2024), adapted from PRISMA [ 22 ]
Bibliographic search flowchart, adapted from PRISMA 2020.
Risk of bias
In this study, the risk of bias assessment was carried out using version 2 of the Cochrane tool for risk of bias in randomized trials (RoB 2). When examining each included study individually, it was observed that all three included studies raised concerns regarding the risk of bias, as illustrated in Fig. 2 :
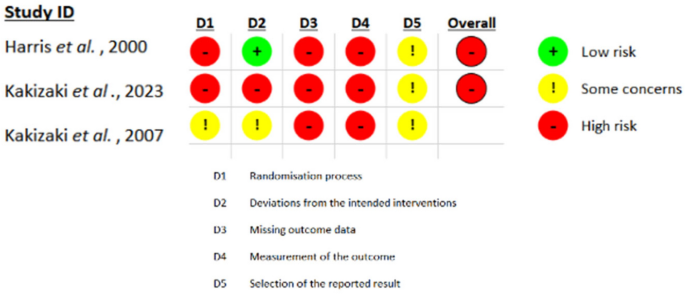
Source: Authors (2024), adapted from the ROB-2 Tool
Individual analysis of bias for each included study.
Given the nature of the intervention in the treatment of orbital floor fractures (blow out), improving the risk of bias is impossible. Orbital floor fractures have complex implications for eye movement, and the variability in surgical techniques, surgeon experience and individual patient characteristics contributes to the possibility of bias in the results of these studies. Therefore, it will not be possible to establish a totally reliable conclusion from this systematic review. The presence of bias can distort the findings and compromise the validity of the conclusions drawn from this analysis. This highlights the importance of adequately addressing and mitigating bias in future studies to ensure an accurate and reliable understanding of the topic in question.
This study critically analyzed the existing literature on the relationship between orbital floor fractures and their consequences for eye movement. The results obtained from the included studies did not provide conclusive evidence establishing a direct relationship between orbital floor fractures and eye movement. Although in the current literature some studies have suggested a possible association, the lack of consensus and the heterogeneity of the results highlight the need for further research to clarify this complex relationship. This review highlights the importance of multidisciplinary approaches and high-quality studies for a more comprehensive understanding of the repercussions of orbital floor fractures on ocular function.
Some studies suggest that blow-out fractures are associated with limited ocular motility and can therefore result in ocular pathologies [ 24 ]. When a fracture occurs in the floor of the orbit, possibly longitudinal rupture of the rectus muscle, vertical diplopia, muscle contusion, scarring within and around the orbital fibrous sheath network, nerve contusion and incarceration within fractures and fibrosis or incarceration involving the muscular fascial network can be common repercussions of trauma [ 25 ]. These complications not only affect visual function, but can also have a significant impact on the patient's quality of life. However, it is necessary to check the methodologies used in such research extensively, so that there are no inconsistencies in the results presented [ 26 , 27 , 28 , 29 ].
In the context of trapdoor fractures of the orbital floor, those that do not involve muscle incarceration generally have a more favorable prognosis in terms of eye movements. However, when muscle incarceration occurs in trapdoor fractures, paralysis of the inferior oblique muscle can contribute to disturbances in ocular motility, in addition to the disturbances caused by connective tissue septa [ 30 ]. Most experts believe that the restriction of motility after blow-out fractures is caused by soft tissue edema and hemorrhage, or by damage to the muscles that control eye movements, such as the inferior rectus, inferior oblique and medial rectus, or even a combination of both, due to the bony fixation of the muscles and fascia [ 31 ]. However, the results of this review revealed a lack of robust evidence to support this claim. The limited methodology of the included studies raises concerns about the reliability of the results. Thus, late motility problems after orbital fractures with or without repair remain poorly understood and challenging to treat, as they resemble other eye movement restrictions, regardless of the underlying cause [ 32 , 33 , 34 , 35 ].
Imaging tests such as Computed Tomography (CT) can be used to analyze the relationship between fractures and ocular motility before surgery in cases of blow-out orbital fractures. Although the use of CT is a relevant way of assessing this type of trauma, there is a bias in its ability to predict the recovery of post-operative motility. Thus, the interactions between bone fragments and soft tissues may not be fully represented by CT images, which can lead to inaccurate inferences about the results of post-surgical ocular motility. Furthermore, the classification of injuries as burst fractures based on CT can be subjective and may not fully reflect the extent of tissue damage or the severity of subsequent fibrosis. Therefore, the relationship between the degree of soft tissue incarceration or displacement and motility outcomes may be more complex than this approach suggests [ 36 , 37 , 38 , 39 ].
In short, there is no concrete evidence that blow out fractures alone can affect the motor function of the ocular nerve, since other factors such as trauma and the surgical intervention itself can also result in neurogenic diplopia. In addition, syndromes can also have an influence on this process. As a result, diplopia can be significantly affected by a number of factors [ 40 , 41 , 42 , 43 ].
After a systematic analysis of the literature and with the results found to compose this systematic review, it is limited to establish a direct relationship between the fracture of the floor of the orbit and repercussions on eye movement.
Data availability
No datasets were generated or analysed during the current study.
Tsyhykalo OV, Kuzniak NB, Dmytrenko RR, Perebyjnis PP, Oliinyk IY, Fedoniuk LY. Features of morphogenesis of the bones of the human orbit. Wiad Lek. 2023;76(1):189–97. https://doi.org/10.36740/WLek202301126 .
Article PubMed Google Scholar
Damasceno RWF, Barbosa JAP, Cortez LRC, Belfort R. Orbital lymphatic vessels: immunohistochemical detection in the lacrimal gland, optic nerve, fat tissue, and extrinsic oculomotor muscles. Arq Bras Oftalmol. 2021;84(3):209–13. https://doi.org/10.5935/0004-2749.20210035 .
Turvey TA, Golden BA. Orbital anatomy for the surgeon. Oral Maxillofac Surg Clin North Am. 2012;24(4):525–36.
Article PubMed PubMed Central Google Scholar
Villalonga JF, Sáenz A, Revuelta Barbero JM, Calandri I, Campero Á. Surgical anatomy of the orbit. A systematic and clear study of a complex structure. Neurocirugia. 2019;30(6):259–67. https://doi.org/10.1016/j.neucir.2019.04.003 . ( English, Spanish ).
Article Google Scholar
Susarla S, Hopper RA, Mercan E. Intact periorbita can prevent post-traumatic enophthalmos following a large orbital blow-out fracture. Craniomaxillofac Trauma Reconstr. 2020;13(1):49–52. https://doi.org/10.1177/1943387520903545 .
Døving M, Lindal FP, Mjøen E, Galteland P. Orbital fractures. Tidsskr Nor Laegeforen. 2022. https://doi.org/10.4045/tidsskr.21.0586 . ( English, Norwegian ).
Oleck NC, Dobitsch AA, Liu FC, Halsey JN, Le TT, Hoppe IC, Lee ES, Granick MS. Traumatic falls in the pediatric population: facial fracture patterns observed in a leading cause of childhood injury. Ann Plast Surg. 2019;82(4S):S195–8. https://doi.org/10.1097/SAP.0000000000001861 .
Article CAS PubMed Google Scholar
Shivakotee S, Menon S, Sham ME, Kumar V, Archana S. Midface fracture pattern in a tertiary care hospital: a prospective study. Natl J Maxillofac Surg. 2022;13(2):238–42. https://doi.org/10.4103/njms.njms_378_21 .
Kono S, Yokota H, Naito M, Vaidya A, Kakizaki H, Kamei M, Takahashi Y. Pressure onto the orbital walls and orbital morphology in orbital floor or medial wall fracture: a 3-dimensional printer study. J Craniofac Surg. 2023;34(6):e608–12. https://doi.org/10.1097/SCS.0000000000009565 .
Kelishadi SS, Zeiderman MR, Chopra K, Kelamis JA, Mundinger GS, Rodriguez ED. Facial fracture patterns associated with traumatic optic neuropathy. Craniomaxillofac Trauma Reconstr. 2019;12(1):39–44. https://doi.org/10.1055/s-0038-1641172 .
Chung SY, Langer PD. Pediatric orbital blowout fractures. Curr Opin Ophthalmol. 2017;28(5):470–6. https://doi.org/10.1097/ICU.0000000000000407 .
Ramponi DR, Astorino T, Bessetti-Barrett CR. Orbital floor fractures. Adv Emerg Nurs J. 2017;39(4):240–7. https://doi.org/10.1097/TME.0000000000000163 .
Kansara A, Doshi H, Shah P, Bathla M, Agrawal N, Gajjar R, Shukla R, Chauhan V. A retrospective study on profile of patients with faciomaxilary fractures in a tertiary care center. Indian J Otolaryngol Head Neck Surg. 2023;75(3):1435–40. https://doi.org/10.1007/s12070-023-03574-y .
Franco VP, Gonçalves GM, Fração OC, Sungaila HYF, Cocco LF, Dobashi ET. Evaluation of the epidemiology of exposed fractures before and during the COVID-19 pandemic. Acta Ortop Bras. 2023;31(4): e268179. https://doi.org/10.1590/1413-785220233104e268179 .
Jain SM, Gehlot N, Kv A, Prasad P, Mehta P, Paul TR, Dupare A, Cvns CS, Rahman S. Ophthalmic complications in maxillofacial trauma: a prospective study. Cureus. 2022;14(8): e27608. https://doi.org/10.7759/cureus.27608 .
Septa D, Newaskar VP, Agrawal D, Tibra S. Etiology, incidence and patterns of mid-face fractures and associated ocular injuries. J Maxillofac Oral Surg. 2014;13(2):115–9. https://doi.org/10.1007/s12663-012-0452-9 .
Zamboni RA, Wagner JCB, Volkweis MR, Gerhardt EL, Buchmann EM, Bavaresco CS. Epidemiological study of facial fractures at the oral and maxillofacial surgery service, Santa Casa de Misericordia Hospital Complex, Porto Alegre-RS-Brazil. Rev Col Bras Cir. 2017;44(5):491–7. https://doi.org/10.1590/0100-69912017005011 .
Joganathan V, Gupta D, Beigi B. Monocular diplopia and nondisplaced inferior rectus muscle on computed tomography in a pediatric pure orbital-floor fracture. J Craniofac Surg. 2018;29(7):1832–3. https://doi.org/10.1097/SCS.0000000000004783 .
Scolozzi P. Reflections on a patient-centered approach to treatment of blow-out fractures: why the wisdom of the pastmust guide our decision-making. J Plast Reconstr Aesthet Surg. 2022;75(7):2268–76. https://doi.org/10.1016/j.bjps.2022.04.034 .
Ellis E 3rd. Orbital trauma. Oral Maxillofac Surg Clin North Am. 2012;24(4):629–48. https://doi.org/10.1016/j.coms.2012.07.006 .
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Explicação e elaboração do PRISMA 2020: orientação atualizada e exemplares para relatar revisões sistemáticas. BMJ. 2021;372(160):1–36. https://doi.org/10.1136/bmj.n160 .
PROSPERO. International prospective register of systematic reviews. Available at: https://www.crd.york.ac.uk/prospero . Accessed 20 Jun 2024.
Ouzzani H, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4 .
Schneider M, Besmens IS, Luo Y, Giovanoli P, Lindenblatt N. Surgical management of isolated orbital floor and zygomaticomaxillary complex fractures with focus on surgical approaches and complications. J Plast Surg Hand Surg. 2020;54(4):200–6. https://doi.org/10.1080/2000656X.2020.1746664 .
Gowda AU, et al. Resolution of vertical gaze following a delayed presentation of orbital floor fracture with inferior rectus entrapment: the contributions. Craniomaxillofac Trauma Reconstr. 2020;13(4):253–9.
Alsaleh F, et al. Clinical correlations of extraocular motility limitation pattern in orbital fracture cases: a retrospective cohort study in a level 1 trauma centre. Orbit. 2023;42(5):487–95.
Iliff N, et al. Mechanisms of extraocular muscle injury in orbital fractures. Plastic Reconstr Surg. 1999;103(3):787–99.
Article CAS Google Scholar
Kashima T, Akiyama H, Kishi S. Longitudinal tear of the inferior rectus muscle in orbital floor fracture. Orbit. 2012;31(3):171–3.
Morax S, Pascal D. Surgical-treatment of oculomotor disturbance resulting from floor fractures. J Francais D Ophtalmol. 1984;7(10):633–47.
CAS Google Scholar
Guerra RC, Pulino BFB, Mendes BC, Pereira RDS, Pinheiro FL, Hochuli-Vieira E. Orbital trapdoor facture in child: more predictable outcomes and less consequences. J Craniofac Surg. 2020;31(5):e469–71. https://doi.org/10.1097/SCS.0000000000006438 .
Düzgün S, Kayahan Sirkeci B. Comparison of post-operative outcomes of graft materials used in reconstruction of blow-out fractures. Ulus Travma Acil Cerrahi Derg. 2020;26(4):538–44. https://doi.org/10.14744/tjtes.2020.80552 .
Kakizaki H, et al. Prognosis of orbital floor trapdoor fractures with or without muscle incarceration. Eur J Plastic Surg. 2007;30:53–6.
Kakizaki H, et al. Incarceration of the inferior oblique muscle branch of the oculomotor nerve in two cases of orbital floor trapdoor fracture. Jpn J Ophthalmol. 2005;49:246–52.
Helveston EM. The relationship of extraocular muscle problems to orbital floor fractures: early and late management. Transactions. Section on ophthalmology. Am Acad Ophthalmol Otolaryngol. 1997;83(4 Pt 1):660–2.
Google Scholar
Reny A, Stricker M. Oculomotoric disturbances after orbital fractures (author’s transl). Klin Monatsbl Augenheilkd. 1973;162(6):750–60.
CAS PubMed Google Scholar
Harris GJ, et al. Correlation of preoperative computed tomography and postoperative ocular motility in orbital blowout fractures. Ophthal Plastic Reconstr Surg. 2000;16(3):179–87.
Harris GJ, et al. Orbital blow-out fractures: correlation of preoperative computed tomography and postoperative ocular motility. Transact Am Ophthalmol Soc. 1998;96:329.
Hong S, Kim J, Baek S. Blowout fracture assessment based on computed tomography and endoscopy: the effectiveness of endoscopy for fracture repair. J Craniofac Surg. 2022;33(4):1008–12. https://doi.org/10.1097/SCS.0000000000008170 .
Felding UNA. Blowout fractures—clinic, imaging and applied anatomy of the orbit. Dan Med J. 2018;65(3):B5459.
PubMed Google Scholar
Ramphul A, Hoffman G. Does preoperative diplopia determine the incidence of postoperative diplopia after repair of orbital floor fracture? An institutional review. J Oral Maxillofac Surg. 2017;75(3):565–75.
Hong S, Choi KE, Kim J, Lee H, Lee H, Baek S. Analysis of patients with blowout fracture caused by baseball trauma. J Craniofac Surg. 2022;33(4):1190–2. https://doi.org/10.1097/SCS.0000000000008492 .
Silva JD, et al. Tratamento de fratura blowout com auxílio de vídeo-cirurgia. Rev Brasil Oftalmol. 2019;78:188–91.
Download references
There was no funding for this research.
Author information
Authors and affiliations.
Health Sciences Center, Federal University of Paraíba (UFPB), João Pessoa, Paraíba, Brazil
Ilan Hudson Gomes de Santana
Centro Universitário de João Pessoa-UNIPÊ, João Pessoa, Paraíba, Brazil
Mayara Rebeca Martins Viana
Paraíba State Employees Health Care Institute - IASS, João Pessoa, Paraíba, Brazil
Julliana Cariry Palhano-Dias
Bauru School of Dentistry, University of São Paulo (FOB-USP), Bauru, São Paulo, Brazil
Osny Ferreira-Júnior & Eduardo Sant’Ana
Oral and Maxillofacial Surgeon, Department of Oral and Maxillofacial Surgery, Hospital Regional of Osasco “Dr. Vivaldo Martins Simões” SUS/SP, Osasco, São Paulo, Brazil
Élio Hitoshi Shinohara
Department of Clinical and Social Dentistry (DCOS), Health Sciences Center, Federal University of Paraíba (UFPB), João Pessoa, Paraíba, Brazil
Eduardo Dias Ribeiro
You can also search for this author in PubMed Google Scholar
Contributions
Conception and planning of the study: Elio Hitoshi Shinohara; Data collection and analysis: Ilan Santana, Mayara Viana, Julliana Pallhano-Dias; Interpretation of results: All the authors contributed to the interpretation of the results obtained from the data analysis, collaborating in the discussion of the findings and the drawing up of well-founded conclusions. Writing the manuscript: Ilan Hudson Gomes de Santana was responsible for the initial writing of the manuscript, while all the co-authors contributed to the writing of the materials and methods, results, discussion and conclusions, ensuring the clarity and cohesion of the text. Critical revision of the content: All the authors carried out critical revisions of the content of the manuscript, incorporating feedback and suggestions from the co-authors and making the necessary adjustments to improve the quality and accuracy of the text. Approval of the final version: All the authors contributed to the review and approval of the final version of the manuscript submitted for publication, ensuring its compliance with the ethical and scientific standards required by the journal.
Corresponding author
Correspondence to Ilan Hudson Gomes de Santana .
Ethics declarations
Ethics approval and consent to participate.
As this is a systematic review, it was not necessary to obtain approval from the research ethics committee to carry out this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
de Santana, I.H.G., Viana, M.R.M., Palhano-Dias, J.C. et al. Orbital floor fracture (blow out) and its repercussions on eye movement: a systematic review. Eur J Med Res 29 , 427 (2024). https://doi.org/10.1186/s40001-024-02023-y
Download citation
Received : 15 May 2024
Accepted : 09 August 2024
Published : 20 August 2024
DOI : https://doi.org/10.1186/s40001-024-02023-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Orbital fractures
- Oculomotor nerve trauma
- Orbit and ocular motility disorders
European Journal of Medical Research
ISSN: 2047-783X
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.13(5); 2023
- PMC10230988

Original research
Evidence-based practice models and frameworks in the healthcare setting: a scoping review, jarrod dusin.
1 Department of Evidence Based Practice, Children’s Mercy Hospitals and Clinics, Kansas City, Missouri, USA
2 Therapeutic Science, The University of Kansas Medical Center, Kansas City, Kansas, USA
Andrea Melanson
Lisa mische-lawson, associated data.
bmjopen-2022-071188supp001.pdf
bmjopen-2022-071188supp002.pdf
No data are available.
The aim of this scoping review was to identify and review current evidence-based practice (EBP) models and frameworks. Specifically, how EBP models and frameworks used in healthcare settings align with the original model of (1) asking the question, (2) acquiring the best evidence, (3) appraising the evidence, (4) applying the findings to clinical practice and (5) evaluating the outcomes of change, along with patient values and preferences and clinical skills.
A Scoping review.
Included sources and articles
Published articles were identified through searches within electronic databases (MEDLINE, EMBASE, Scopus) from January 1990 to April 2022. The English language EBP models and frameworks included in the review all included the five main steps of EBP. Excluded were models and frameworks focused on one domain or strategy (eg, frameworks focused on applying findings).
Of the 20 097 articles found by our search, 19 models and frameworks met our inclusion criteria. The results showed a diverse collection of models and frameworks. Many models and frameworks were well developed and widely used, with supporting validation and updates. Some models and frameworks provided many tools and contextual instruction, while others provided only general process instruction. The models and frameworks reviewed demonstrated that the user must possess EBP expertise and knowledge for the step of assessing evidence. The models and frameworks varied greatly in the level of instruction to assess the evidence. Only seven models and frameworks integrated patient values and preferences into their processes.
Many EBP models and frameworks currently exist that provide diverse instructions on the best way to use EBP. However, the inclusion of patient values and preferences needs to be better integrated into EBP models and frameworks. Also, the issues of EBP expertise and knowledge to assess evidence must be considered when choosing a model or framework.
STRENGTHS AND LIMITATIONS OF THIS STUDY
- Currently, no comprehensive review exists of evidence-based practice (EBP) models and frameworks.
- Well-developed models and frameworks may have been excluded for not including all five steps of original model for EBP.
- This review did not measure the quality of the models and frameworks based on validated studies.
Introduction
Evidence-based practice (EBP) grew from evidence-based medicine (EBM) to provide a process to review, translate and implement research with practice to improve patient care, treatment and outcomes. Guyatt 1 coined the term EBM in the early 1990s. Over the last 25 years, the field of EBM has continued to evolve and is now a cornerstone of healthcare and a core competency for all medical professionals. 2 3 At first, the term EBM was used only in medicine. However, the term EBP now applies to the principles of other health professions. This expansion of the concept of EBM increases its complexity. 4 The term EBP is used for this paper because it is universal across professions.
Early in the development of EBP, Sackett 5 created an innovative five-step model. This foundational medical model provided a concise overview of the process of EBP. The five steps are (1) asking the question, (2) acquiring the best evidence, (3) appraising the evidence, (4) applying the findings to clinical practice and (5) evaluating the outcomes of change. Other critical components of Sackett’s model are considering patient value and preferences and clinical skills with the best available evidence. 5 The influence of this model has led to its integration and adaption into every field of healthcare. Historically, the foundation of EBP has focused on asking the question, acquiring the literature and appraising the evidence but has had difficulty integrating evidence into practice. 6 Although the five steps appear simple, each area includes a vast number of ways to review the literature (eg, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), Newcastle-Ottawa Scale) and entire fields of study, such as implementation science, a field dedicated to implementing EBP. 7 8 Implementation science can be traced to the 1960s with Everett Rogers’ Diffusion of Innovation Theory and has grown alongside EBP over the last 25 years. 7 9
One way to manage the complexity of EBP in healthcare is by developing EBP models and frameworks that establish strategies to determine resource needs, identify barriers and facilitators, and guide processes. 10 EBP models and frameworks provide insight into the complexity of transforming evidence into clinical practice. 11 They also allow organisations to determine readiness, willingness and potential outcomes for a hospital system. 12 EBP can differ from implementation science, as EBP models include all five of Sackett’s steps of EBP, while the non-process models of implementation science typically focus on the final two steps. 5 10 There are published scoping reviews of implementation science, 13 however, no comprehensive review of EBP models and frameworks currently exists. Although there is overlap of EBP, implementation science and knowledge translation models and frameworks 10 14 the purpose of the scoping review was to explore how EBP models and frameworks used in healthcare settings align with the original EBP five-step model.
A scoping review synthesises findings across various study types and provides a broad overview of the selected topic. 15 The Arksey and O’Malley method and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-ScR) procedures guided this review (see online supplemental PRISMA-ScR checklist ). 15 16 The primary author established the research question and inclusion and exclusion criteria before conducting the review. An a priori protocol was not pre-registered. One research question guided the review: Which EBP models and frameworks align with Sackett’s original model?
Supplementary data
Eligibility criteria.
To be included in the review, English language published EBP models and frameworks needed to include the five main steps of EBP (asking the question, acquiring the best evidence, appraising the evidence, applying the findings to clinical practice and assessing the outcomes of change) based on Sackett’s model. 5 If the models or frameworks involved identifying problems or measured readiness for change, the criteria of ‘asking the question’ was met. Exclusions included models or frameworks focused on one domain or strategy (eg, frameworks focused on applying findings). Also, non-peer-reviewed abstracts, letters, editorials, opinion articles, and dissertations were excluded.
Search and selection
To identify potential studies, a medical librarian searched the databases from January 1990 to April 2022 in MEDLINE, EMBASE and Scopus in collaboration with the primary author. The search was limited to 1990 because the term EBP was coined in the early 90s. The search strategy employed the following keywords: ‘Evidence-Based Practice’ OR ‘evidence based medicine’ OR ‘evidence-based medicine’ OR ‘evidence based nursing’ OR ‘evidence-based nursing’ OR ‘evidence based practice’ OR ‘evidence-based practice’ OR ‘evidence based medicine’ OR ‘evidence-based medicine’ OR ‘evidence based nursing’ OR ‘evidence-based nursing’ OR ‘evidence based practice’ OR ‘evidence-based practice’ AND ‘Hospitals’ OR ‘Hospital Medicine’ OR ‘Nursing’ OR ‘Advanced Practice Nursing’ OR ‘Academic Medical Centers’ OR ‘healthcare’ OR ‘hospital’ OR ‘healthcare’ OR ‘hospital’ AND ‘Models, Organizational’ OR ‘Models, Nursing’ OR ‘framework’ OR ‘theory’ OR ‘theories’ OR ‘model’ OR ‘framework’ OR ‘theory’ OR ‘theories’ OR ‘model’. Additionally, reference lists in publications included for full-text review were screened to identify eligible models and frameworks (see online supplemental appendix A for searches).
Selection of sources of evidence
Two authors (JD and AM) independently screened titles and abstracts and selected studies for potential inclusion in the study, applying the predefined inclusion and exclusion criteria. Both authors then read the full texts of these articles to assess eligibility for final inclusion. Disagreement between the authors regarding eligibility was resolved by consensus between the three authors (JD, AM and LM-L). During the selection process, many models and frameworks were found more than once. Once a model or framework article was identified, the seminal article was reviewed for inclusion. If models or frameworks had been changed or updated since the publication of their seminal article, the most current iteration published was reviewed for inclusion. Once a model or framework was identified and verified for inclusion, all other articles listing the model or framework were excluded. This scoping review intended to identify model or framework aligned with Sackett’s model; therefore, analysing every article that used the included model or framework was unnecessary (see online supplemental appendix B for tracking form).
Data extraction and analysis
Data were collected on the following study characteristics: (1) authors, (2) publication year, (3) model or framework and (4) area(s) of focus in reference to Sackett’s five-step model. After initial selection, models and frameworks were analysed for key features and alignment to the five-step EBP process. A data analysis form was developed to map detailed information (see online supplemental appendix C for full data capture form). Data analysis focused on identifying (1) the general themes of the model or frameworks, and (2) any knowledge gaps. Data extraction and analysis were done by the primary author (JD) and verified by one other author (AM). 15
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
The search identified 6523 potentially relevant references (see figure 1 ). Following a review of the titles and abstracts, the primary author completed a more detailed screening of 37 full papers. From these, 19 models and frameworks were included. Table 1 summarises the 19 models and frameworks. Of the 19 models and frameworks assessed and mapped, 15 had broad target audiences, including healthcare or public health organisations or health systems. Only five models and frameworks included a target audience of individual clinicians (eg, physicians and nurses). 17–22

Retrieval and selection process.
Models and frameworks organised by integration of patient preferences and values
| Name | Steps of model or framework | General themes | Knowledge gaps | ||
| Patient values incorporated into model | |||||
| Iowa Model | 1. Question development 2. Searches, appraises and synthesises the literature 3. If literature is lacking, conduct research | 4.Develop, enact and appraise a pilot solution 5. If successful, implement across organisation 6. If unsuccessful, restart process | |||
| Monash Partners Learning Health Systems Framework | 1. Stakeholder-driven 2. Engage the people 3. Identify priorities 4. Research evidence 5. Evidence-based information 6. Evidence synthesis | 7. Data-derived evidence 8. Data/information systems 9. Benchmarking 10. Implementation evidence 11. Implementation 12. Healthcare improvement | |||
| ARCC | 1. Assess the healthcare organisation for readiness for change 2. Identify potential and actual barriers and facilitators 3. Identify EBP champions | 4. Implement evidence into practice 5. Evaluate EBP outcomes | |||
| The Clinical Scholar Model | 1. Observation 2. Analysis 3. Synthesis | 4. Application/ evaluation 5. Dissemination | |||
| JBI | 1. Global Health 2. Evidence generation 3. Evidence synthesis | 4. Evidence (knowledge) transfer 5. Evidence implementation | |||
| CETEP | 1. Define the clinical practice question 2. Assess the critical appraisal components 3. Plan the implementation | 4. Implement the practice change 5. Evaluate the practice change | |||
| Johns Hopkins | 1. Practice question: EBP question is identified 2. Evidence: the team searches, appraises, rates the strength of evidence 3. Translation: feasibility, action plan and change implemented and evaluated | ||||
| Patient values discussed, not incorporated into models/frameworks | |||||
| Stetler Model | 1. Question development includes project context 2. Identify the relevance of evidence sources and quality 3. Summarise evidence 4. Develop a plan 5. Identify/collect data outcomes to evaluate effectiveness of plan | ||||
| KTA | 1. Identify problems and begin searching for evidence 2. Adapt knowledge to local context 3. Identify barriers 4. Select, adapt, and implement | 5. Monitor implanted knowledge 6. Evaluate outcomes related to knowledge use 7. Sustain appropriate knowledge use | |||
| EBMgt | 1. Asking; acquiring; appraising; aggregating; applying; and assessing 2. Predictors; barriers; training organisations; and research institutes | ||||
| St Luke’s | 1. Area of interest 2. Collect the best evidence 3. Critically appraise the evidence | 4. Integrate the evidence, clinical skill and patient preferences/values 5. Evaluate the practice change | |||
| The I3 Model for Advancing Quality Patient Centred Care | 1. Inquiry 2. Improvement 3. Innovation | 4. Inquiry encompasses research 5. Improvement includes quality improvement projects 6. Innovation is discovery studies and best evidence projects | |||
| Model for Change to Evidence Based Practice | 1. Identify need to change practice 2. Approximate problem with outcomes 3. Summarise best scientific evidence 4. Develop plan for changing practice | 5. Implement and evaluate change (pilot study) 6. Integrate and maintain change in practice 7. Monitor implementation | |||
| Patient values not discussed | |||||
| Evidence-Based Public Health | 1. Community assessment 2. Quantify the issue 3. Develop statement of the issue 4. Determine what is known evidence | 5. Develop and prioritise programme and policy options 6. Develop an action plan 7. Evaluate the programme or policy | |||
| ACE Star Model | 1. Discovery: Searching for new knowledge 2. Evidence Summary: Synthesise the body of research knowledge 3. Translation: Provide clinicians with a practice document 4. Integration: Changed through formal and informal channels 5. Evaluation: EBP outcomes are evaluated | ||||
| An Evidence Implementation Model for Public Health Systems | Not a linear model 1. Circle 1 Evidence implementation target 2. Circle 2 Actors involved in implementation | 3. Circle 3 Knowledge transfer 4. Circle 4 Barriers and facilitators | |||
| San Diego 8A’s EBP Model | 1. Assessing a clinical or practice problem 2. Asking a clinical question in a PICO format 3. Acquiring existing sources of evidence 4. Appraising the levels of evidence | 5.Applying the evidence to a practice change 6. Analysing the results of the change 7. Advancing the practice change through dissemination 8. Adopting the practice of sustainability over time | |||
| Tyler Collaborative Model for EBP | Phase one: unfreezing 1. Building relationships 2. Diagnosing the problem 3. Acquiring resources Phase two: moving 1. Choosing the solution 2. Gaining acceptance | Phase three: refreezing 1. Stabilisation | |||
| The Practice Guidelines Development Cycle | 1. Select/frame clinical problem 2. Generate recommendations 3. Ratify recommendations 4. Formulate practice guideline | 5. Independent review 6. Negotiate practice policies 7. Adopt guideline policies 8. Scheduled review | |||
EBP, evidence-based practice.
Asking the question
All 19 models and frameworks included a process for asking questions. Most focused on identifying problems that needed to be addressed on an organisational or hospital level. Five used the PICO (population, intervention, comparator, outcome) format to ask specific questions related to patient care. 19–25
Acquiring the evidence
The models and frameworks gave basic instructions on acquiring literature, such as ‘conduct systematic search’ or ‘acquire resource’. 20 Four recommended sources from previously generated evidence, such as guidelines and systematic reviews. 6 21 22 26 Although most models and frameworks did not provide specifics, others suggested this work be done through EBP mentors/experts. 20 21 25 27 Seven models included qualitative evidence in the use of evidence, 6 19 21 24 27–29 while only four models considered the use of patient preference and values as evidence. 21 22 24 27 Six models recommended internal data be used in acquiring information. 17 20–22 24 27
Assessing the evidence
The models and frameworks varied greatly in the level of instruction provided in assessing the best evidence. All provided a general overview in assessing and grading the evidence. Four recommended this work be done by EBP mentors and experts. 20 25 27 30 Seven models developed specific tools to be used to assess the levels of evidence. 6 17 21 22 24 25 27
Applying the evidence
The application of evidence also varied greatly for the different models and frameworks. Seven models recommended pilot programmes to implement change. 6 21–25 31 Five recommended the use of EBP mentors and experts to assist in the implementation of evidence and quality improvement as a strategy of the models and frameworks. 20 24 25 27 Thirteen models and frameworks discussed patient values and preferences, 6 17–19 21–27 31 32 but only seven incorporated this topic into the model or framework, 21–27 and only five included tools and instructions. 21–25 Twelve of the 20 models discussed using clinical skill, but specifics of how this was incorporated was lacking in models and frameworks. 6 17–19 21–27 31
Evaluating the outcomes of change
Evaluation varied among the models and frameworks, but most involved using implementation outcome measures to determine the project’s success. Five models and frameworks provide tools and in-depth instruction for evaluation. 21 22 24–26 Monash Partners Learning Health Systems provided detailed instruction on using internal institutional data to determine success of application. 26 This framework uses internal and external data along with evidence in decision making as a benchmark for successful implementation.
EBP models and frameworks provide a process for transforming evidence into clinical practice and allow organisations to determine readiness and willingness for change in a complex hospital system. 12 The large number of models and frameworks complicates the process by confusing what the best tool is for healthcare organisations. This review examined many models and frameworks and assessed the characteristics and gaps that can better assist healthcare organisations to determine the right tool for themselves. This review identified 19 EBP models and frameworks that included the five main steps of EBP as described by Sackett. 5 The results showed that the themes of the models and frameworks are as diverse as the models and frameworks themselves. Some are well developed and widely used, with supporting validation and updates. 21 22 24 27 One such model, the Iowa EBP model, has received over 3900 requests for permission to use it and has been updated from its initial development and publication. 24 Other models provided tools and contextual instruction such as the Johns Hopkin’s model which includes a large number of supporting tools for developing PICOs, instructions for grading literature and project implementation. 17 21 22 24 27 By contrast, the ACE Star model and the An Evidence Implementation Model for Public Health Systems only provide high level overview and general instructions compared with other models and frameworks. 19 29 33
Gaps in the evidence
A consistent finding in research of clinician experience with EBP is the lack of expertise that is needed to assess the literature. 24 34 35 The models and frameworks reviewed demonstrated that the user must possess the knowledge and related skills for this step in the process. The models and frameworks varied greatly in the level of instruction to assess the evidence. Most provided a general overview in assessing and grading the evidence, though a few recommended that this work be done by EBP mentors and experts. 20 25 27 ARCC, JBI and Johns Hopkins provided robust tools and resources that would require administrative time and financial support. 21 22 27 Some models and frameworks offered vital resources or pointed to other resources for assessing evidence, 24 but most did not. While a few used mentors and experts to assist with assessing the literature, a majority did not address this persistent issue.
Sackett’s five-step model included another important consideration when implementing EBP: patient values and preferences. One criticism of EBP is that it ignores patient values and preferences. 36 Over half of the models and frameworks reported the need to include patient values and preferences, but the tools, instruction or resources for including them were limited. The ARCC model integrates patient preferences and values into the model, but it is up to the EBP mentor to accomplish this task. 37 There are many tools for assessing evidence, but few models and frameworks provide this level of guidance for incorporating patient preference and values. The inclusion of patient and family values and preferences can be misunderstood, insincere, and even tokenistic but without it there is reduced chance of success of implementation of EBP. 38 39
Strengths and limitations
Similar to other well-designed scoping reviews, the strengths of this review include a rigorous search conducted by a skilled librarian, literature evaluation by more than one person, and the utilisation of an established methodological framework (PRISMA-ScR). 14 15 Additionally, utilising the EBP five-step models as a point of alignment allows for a more comprehensive breakdown and established reference points for the reviewed models and frameworks. While scoping reviews have been completed on implementation science and knowledge translation models and framework, to our knowledge, this is the first scoping review of EBP models and frameworks. 13 14 Limitations of the study include that well-developed models and frameworks may have been excluded for not including all five steps. 40 For example, the Promoting Action on Research Implementation in Health Services (PARIHS) framework is a well-developed and validated implementation framework but did not include all five steps of an EBP model. 40 Also, some models and frameworks have been studied and validated over many years. It was beyond the scope of the review to measure the quality of the models and frameworks based on these other validated studies.
Implications and future research
Healthcare organisations can support EBP by choosing a model or framework that best suits their environment and providing clear guidance for implementing the best evidence. Some organisations may find the best fit with the ARCC and the Clinical Scholars Model because of the emphasis on mentors or the Johns Hopkins model for its tools for grading the level of evidence. 21 25 27 In contrast, other organisations may find the Iowa model useful with its feedback loops throughout its process. 24
Another implication of this study is the opportunity to better define and develop robust tools for patient and family values and preferences within EBP models and frameworks. Patient experiences are complex and require thorough exploration, so it is not overlooked, which is often the case. 39 41 The utilisation of EBP models and frameworks provide an opportunity to explore this area and provide the resources and understanding that are often lacking. 38 Though varying, models such as the Iowa Model, JBI and Johns Hopkins developed tools to incorporate patient and family values and preferences, but a majority of the models and frameworks did not. 21 22 24 An opportunity exists to create broad tools that can incorporate patient and family values and preferences into EBP to a similar extent as many of the models and frameworks used for developing tools for literature assessment and implementation. 21–25
Future research should consider appraising the quality and use of the different EBP models and frameworks to determine success. Additionally, greater clarification on what is considered patient and family values and preferences and how they can be integrated into the different models and frameworks is needed.
This scoping review of 19 models and frameworks shows considerable variation regarding how the EBP models and frameworks integrate the five steps of EBP. Most of the included models and frameworks provided a narrow description of the steps needed to assess and implement EBP, while a few provided robust instruction and tools. The reviewed models and frameworks provided diverse instructions on the best way to use EBP. However, the inclusion of patient values and preferences needs to be better integrated into EBP models. Also, the issues of EBP expertise to assess evidence must be considered when selecting a model or framework.
Supplementary Material
Acknowledgments.
We thank Keri Swaggart for completing the database searches and the Medical Writing Center at Children's Mercy Kansas City for editing this manuscript.
Contributors: All authors have read and approved the final manuscript. JD conceptualised the study design, screened the articles for eligibility, extracted data from included studies and contributed to the writing and revision of the manuscript. LM-L conceptualised the study design, provided critical feedback on the manuscript and revised the manuscript. AM screened the articles for eligibility, extracted data from the studies, provided critical feedback on the manuscript and revised the manuscript. JD is the guarantor of this work.
Funding: The article processing charges related to the publication of this article were supported by The University of Kansas (KU) One University Open Access Author Fund sponsored jointly by the KU Provost, KU Vice Chancellor for Research, and KUMC Vice Chancellor for Research and managed jointly by the Libraries at the Medical Center and KU - Lawrence
Disclaimer: No funding agencies had input into the content of this manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Ethics statements, patient consent for publication.
Not applicable.
A Comprehensive Review of the Non-operative Management of Traumatic Rib Fractures
- Anesthesia for Trauma (TE Grissom, Section Editor)
- Open access
- Published: 22 August 2024
Cite this article
You have full access to this open access article

- Kaveh Hemati 1 ,
- Andrew T. Gray 1 &
- Ashish Agrawal 1
Purpose of Review
This review summarizes current literature on the non-operative management of traumatic rib fractures, including risk assessment scores, respiratory therapy, and multimodal and regional analgesia.
Recent Findings
Rib fractures are associated with significant morbidity and mortality, especially in elderly patients. Risk assessment scores, such as the Pain Inspiration Cough (PIC) score, allow for early identification and dynamic assessment of those at risk for ICU admission or increased length of stay. Incentive spirometry is both prognostic and therapeutic for patients with rib fractures, while high flow nasal cannula and non-invasive ventilation strategies lack robust evidence. Multimodal analgesia regimens have been associated with significant reductions in opioid exposure and pain scores. Epidural and regional anesthesia remain common analgesic modalities to decrease unplanned intubation and ICU admission especially in elderly patients.
Optimizing pulmonary hygiene and analgesia regimens remain the primary evidence-based goals of management for patients with rib fractures.
Similar content being viewed by others
Analgesia for rib fractures: a narrative review

Traumatic Rib Fracture in the Absence of Flail Chest: Conservative Therapy or Surgical Fixation?
Treatments for blunt chest trauma and their impact on patient outcomes and health service delivery.
Avoid common mistakes on your manuscript.
Introduction
In the United States alone, rib fractures affect over 40,000 people per year, resulting in a cost of over $469 million per year [ 1 ]. Although rib fractures can be atraumatic, most rib fractures are caused by either penetrating or blunt chest trauma. In fact, the most common presenting injury related to blunt chest trauma is rib fractures, occurring in nearly two-thirds of these patients [ 2 , 3 , 4 ]. The clinical importance of rib fractures stems from their association with significant morbidity (48% complication rate) and mortality (22% for older adults) [ 4 , 5 ]. Complications of rib fractures include increased rates of pneumonia, ventilator days, and intensive care unit (ICU) and hospital length of stay (HLOS) [ 6 ]. Patients with an increased number of fractures, older age, and polytrauma have increased morbidity and mortality [ 7 ].
Given the impact rib fractures have on our healthcare system, both in terms of patient morbidity and mortality and the overall burden of hospital care costs, recent studies have aimed to risk stratify patients, identify complications early, and develop management strategies to mitigate the negative effects. In this review, we will examine the associated complications of traumatic rib fractures and summarize evidence and options for risk assessment and triage, respiratory management, and pain management of patients with traumatic rib fractures.
Complications of Rib Fractures
The morbidity and mortality associated with rib fractures can be linked to the direct and indirect effects of the injury on the pulmonary system.
Directly, the significant blunt force required to fracture ribs and the associated energy transfer can result in damage to the underlying tissues, including the pleura and the lung parenchyma. This can lead to sequela such as pneumothorax, hemothorax, or pulmonary contusion [ 8 , 9 ]. Direct damage to the alveolar capillary membrane complex can lead to bleeding and edema, which can impair gas exchange due to intra-pulmonary shunting and reduced compliance. These sequelae can lead to adverse effects on oxygenation and ventilation, which can require higher levels of respiratory support including mechanical ventilation.
Indirectly, rib fractures can cause significant pain that can limit respiratory function and mobility. Pain in the thoracic area may limit chest wall excursion and lead to reduced tidal volumes and an ineffective cough, which contributes to inadequate secretion clearance and atelectasis. In addition, rib fracture pain can reduce the ability of patients to mobilize, further compounding atelectasis [ 10 ]. The limited tidal volumes, atelectasis, and inadequate secretion clearance all contribute to developing pneumonia [ 9 ].
Most significantly, complications of rib fractures are higher in patients over 65 years old. While pneumonia and mortality rates increase in all age groups with a greater number of rib fractures, it increases even more in elderly patients [ 11 ]. Pneumonia rates are 31% in older patients versus 17% in younger patients [ 11 ]. Rib fractures are also associated with long-term pain and disability with only approximately 59% of patients returning to work at 6 months after injury [ 12 , 13 ]. Mortality, irrespective of age, has been estimated to be between 10–12%; for patients hospitalized, this rate increases with each additional rib fractured, approaching > 40% if > 6 ribs are fractured [ 8 , 9 , 11 ]. Deaths usually occur because of multi-organ failure provoked by respiratory insufficiency and pneumonia [ 8 , 14 ].
Because of these resulting issues, the primary goals of managing patients with rib fractures are to identify those most at risk of decompensation and to direct efforts at optimizing oxygenation and ventilation, pulmonary hygiene, early mobility, and effective analgesia regimens [ 2 ].
Risk Assessment and Scoring Systems
Although early triage and standardized care pathways have been shown to reduce complications such as length of stay, pneumonia, and mortality, there currently is no single standardized scoring system or care pathway that has widespread use [ 15 , 16 ]. The scoring systems that have been developed are used to assist in the evaluation of patients, guide treatment decisions, and aid in risk stratifying and prognosticating outcomes in patients with rib fractures.
The three most common scoring systems are the Rib Fracture Score (RFS), Chest Trauma Score (CTS), and RibScore (RS). All three scoring systems have been shown to have moderate discrimination for predicting complications and outcomes [ 16 ]. However, these scores provide a one-time static score and do not vary based on changes in the patient’s clinical status. There are dynamic clinical scoring systems, such as the Pain Inspiration Cough (PIC) score, that may be utilized in serial patient assessments to assist with level of care triage and predicting morbidity over time [ 15 , 17 ]. The newer Sequential Clinical Assessment of Respiratory Function (SCARF) score is another dynamic scoring system that is currently utilized by one academic center. Although we do not describe it here, we recognize its potential value as a dynamic scoring system [ 15 ].
Here we describe the common scoring systems that exist (Table 1 ). Although each institution may use different scoring systems, we do recommend the use of a scoring system and/or evidence-based care pathway to guide the management of patients with rib fractures as this has been shown to be associated with reduced mortality [ 4 ].
Rib Fracture Score (RFS)
The RFS was retrospectively developed by analyzing a large population of trauma patients previously studied in the literature [ 18 , 19 ]. It is calculated as the number of total fractures of the ribs (2 breaks in a single rib counts for 2) multiplied by the number sides affected (1 or 2) plus an age factor (51–60 = 1, 61–70 = 2, 71–80 = 3, > 80 = 4), and it was developed to assess the need for respiratory support, mobilization, and pain management [ 16 , 18 ]. Initially, an RFS score > 6 was suggested as a cutoff to have specific care implemented; however, several validation studies have shown a weak correlation between RFS and hospital length of stay (HLOS) and ICU length of stay (ICULOS) [ 16 , 18 , 20 ]. A retrospective cohort study also showed RFS had poor predictive value for mortality, pneumonia, and rate of tracheostomy for all patients with rib fractures [ 16 ]. However, when analyzed by age group, RFS > 8 in patients 65 years and older had higher mortality, higher injury severity scores (ISS), longer HLOS, longer ICULOS, and higher rates of pneumonia [ 16 ]. Although conflicting data exist for RFS, it may be a valuable tool for geriatric patients.
Chest Trauma Score (CTS)
The CTS was developed to identify patients at risk for morbidity and mortality at admission [ 21 ]. It is calculated by the summation of points from several categories: age, number of ribs fractured, number of sides affected, and pulmonary contusion severity defined by a radiologist [ 16 , 21 , 22 ]. The initial study found different cut points to be associated with distinct morbidities: CTS > 7 associated with higher mortality, ICU admission, intubation; CTS > 5 associated with longer HLOS and longer duration of mechanical ventilation. When analyzed by age group, CTS > 6 in patients 65 and older had higher mortality, higher ISS, longer HLOS, longer ICULOS, longer duration of mechanical ventilation, higher rates of tracheostomies, and higher rates of pneumonia [ 16 ]. Although cut points for CTS and associated morbidities have varied, several validation studies confirm that higher CTS scores predict patients at risk for complications from rib fractures, and thus CTS is a valuable tool to guide early implementation of treatment strategies [ 16 , 22 ].
RibScore (RS)
The RS is a radiographic rib fracture score based on chest computed tomography (CT) [ 23 ]. It allocates points for each of six radiographic variables: six or more rib fractures, bilateral fractures, flail chest, three or more severely displaced fractures, fracture of the first rib, and at least one fracture in all segmental locations [ 23 ]. The initial study confirmed associations between each individual RS variable and total RS with pneumonia, respiratory failure, and tracheostomy [ 23 ]. RS of 4 or higher had greater than 90% specificity for pneumonia, respiratory failure, and tracheostomy [ 16 , 23 ]. When an age factor was added, there was interestingly no increased predictability of RS on morbidities. A recent retrospective cohort study found that both geriatric and non-geriatric patients with RS > 1 had higher mortality, higher ISS, longer HLOS, longer ICULOS, longer duration of mechanical ventilation, higher rates of tracheostomies, and higher rates of pneumonia [ 16 ]. Although data exist for the usage of RS in rib fracture patients, it is recommended that RS be used for the selective assessment of severely injured patients with high ISS values given that its specificity increases with higher scores.
Pain Inspiration Cough (PIC) Score
The PIC score was developed by clinicians as an easy-to-implement, dynamic scoring system that could prognosticate and guide management in patients with rib fractures over time [ 17 , 24 ]. The PIC score is calculated from the summation of three individual category scores (pain, inspiration, cough) to obtain a composite score that can range from 3 to 10 [ 17 ]. During its first implementation, a PIC score of 7 or less was associated with a 57% reduction in unanticipated transfer to a higher level of care for respiratory status decline; HLOS was reduced by 0.7 days and discharge to home improved by 13% [ 17 ]. The PIC score has subsequently been adopted by many institutions and continues to be utilized as a dynamic tool that can not only to inform where to admit patients, but also to determine when to downgrade a patient [ 12 , 24 ]. A recent retrospective cohort study found that a PIC score 7 or lower was highly associated with ICU admission and a prolonged HLOS [ 24 ]. Interestingly, the PIC score cutoff of 7 was a moderate independent predictor of ICULOS > 48 h and was not associated with any particular injury pattern or preinjury comorbidity burden [ 24 ]. The developers of the PIC score have recently liberalized their triage criteria for ICU admission (PIC score 5 or lower), but continue to use PIC score of higher than 7 to guide discharge from the ICU [ 25 ]. Although utilized by many institutions currently as a useful dynamic scoring system, there are discrepancies with PIC score cutoffs to determine ICU admission or downgrades to floor and thus further research is necessary to determine optimal cutoffs. However, we do see great value in the use of the PIC score as its ongoing use requires vigilance from care team members including nursing and respiratory therapy, which will only pay dividends in the early detection and mitigation of respiratory failure emergencies.
Monitoring and Respiratory Management
Given that morbidity and mortality associated with rib fractures stem from issues within the pulmonary system, it is prudent to focus on preventing pulmonary complications and addressing them as they arise. To that end, respiratory parameter monitoring and management is key to successfully caring for patients with rib fractures.
Level of Care / Monitoring
All patients with rib fractures admitted to the hospital should have continuous pulse oximetry available regardless of level of care. ICU admission is recommended for older adults (> 65 years) with three or more rib fractures as this lowers morbidity and mortality [ 4 , 26 , 27 , 28 ]. Scoring systems described earlier can be utilized to risk stratify patients who do not meet these criteria, and institution-specific monitoring guidelines should be established based on a patient’s level of care.
Incentive Spirometry (IS)
Bedside assessments using incentive spirometry (IS) are standard of care as IS can be both therapeutic and prognostic. Therapeutically, IS assists with lung expansion and reduces atelectasis. A recent randomized controlled trial demonstrated that the utilization of IS reduced pulmonary complications, including atelectasis, hemothorax, and interventions such as thoracostomy, in patients with traumatic rib fractures [ 29 ].
Prognostically, IS volume is a predictor of complications. A prospective case-series suggested that low IS volume (< 500 ml) at admission was associated with higher rates of acute respiratory failure [ 30 ]. A retrospective study found that the relative risk of pulmonary complications was 3.3 in patients with incentive spirometry volume < 1000 ml after rib fractures [ 31 ]. Given its minimal adverse effects, low cost, and good therapeutic tolerance, we recommend the use of IS for all patients with rib fractures.
Non-invasive Oxygenation / High Flow Nasal Canula (HFNC)
High flow nasal canula (HFNC) has had increased usage in adults for the treatment and prevention of hypoxemic respiratory failure, but there is limited data surrounding HFNC specifically in patients with rib fractures. A retrospective study of blunt chest trauma patients admitted to the ICU showed an intubation rate of 18% in patients receiving HFNC and that a delay to first initiation of HFNC was correlated with increased HLOS and ICULOS [ 32 ]. One randomized controlled trial compared the use of HFNC and venturi mask in patients with rib fractures and high-risk features and found no statistically significant difference in those requiring mechanical invasive/non-invasive ventilation or unplanned admission to the ICU [ 33 ]. An observational study compared patients with three or more rib fractures receiving HFNC at any location to a historical control group when HFNC was available only in the ICU and found no significant differences in HLOS, mechanical ventilation, or mortality between the study and control group, but 27% of patients in the study group avoided ICU admission entirely [ 34 ]. Although a paucity of quality evidence exists regarding HFNC and rib fractures, we do recommend its use for hypoxemia as there is low risk and potential benefit.
Non-invasive Ventilation / Continuous Positive Airway Pressure (CPAP)
Non-invasive ventilation, such as continuous positive airway pressure (CPAP), has been more commonly studied than HFNC in the blunt chest trauma population [ 35 , 36 , 37 ]. Historic studies showed that CPAP combined with regional analgesia have decreased rates of pneumonia and that CPAP combined with patient-controlled analgesia have lower mortality rates and decreased nosocomial infection rates compared with mechanical ventilation in patients with blunt chest trauma [ 35 , 36 ]. In a recent randomized study, hypoxemic blunt chest trauma patients assigned to receive CPAP had 40% lower intubation rates and reduced overall HLOS by 7 days compared to patients receiving HFNC [ 36 ]. Given these studies, expert practice guidelines currently recommended the use of non-invasive ventilation for older adults (> 65 years) who have three or more rib fractures [ 4 , 38 ].
Pain Management
Systemic opioid analgesics were historically the mainstay of pain management in patients with rib fractures; however, given the significant adverse effects of opioids (respiratory depression, nausea, tolerance and dependence), the core of pain management has shifted to multimodal therapy [ 9 , 13 ]. Multimodal analgesia regimens rely on synergistic combinations of opioid and non-opioid medications and regional anesthesia in an attempt to decrease doses and reduce adverse drug reactions for any individual medication [ 39 ]. A few retrospective cohort studies of trauma patients demonstrated that the implementation of a multimodal pain regimen was associated with significant reductions in opioid exposure, opioid prescriptions at discharge, and a modest reduction in patient-reported Numerical Rating Scale (NRS) pain scores [ 40 , 41 ]. While some of the evidence shown below for each individual modality may be mixed, we continue to advocate for the use of multimodal regimens for rib fracture patients tailored to the risks and benefits of the local patient population.
Systemic Non-Opioid Analgesics
Acetaminophen.
Acetaminophen has become a mainstay of multimodal analgesia protocols, but there have been few studies to specifically look at its utility in rib fracture patients. In a randomized double-blinded clinical trial in patients with rib fractures, pain severity was compared between those who received intravenous acetaminophen and those who received intravenous morphine, and there were no significant differences in efficacy for relieving rib fracture pain or side effects [ 42 ]. A prospective randomized controlled trial of elderly patients with rib fractures compared oral acetaminophen to intravenous acetaminophen and found no difference in pain reduction scores, mortality, HLOS, or the development of pneumonia [ 43 ].
Non-steroidal Anti-inflammatory Drugs (NSAIDs)
The efficacy of NSAIDs for rib fractures has been infrequently studied. A matched retrospective cohort study suggested oral morphine equivalent (OME) totals were less at 7 days in the intravenous ibuprofen group compared to routine care in rib fracture patients [ 44 ]. Although no new strong evidence exists regarding NSAID efficacy in rib fracture patients, many providers historically have had reservations regarding the usage of NSAIDs for posttraumatic analgesia due to concerns about risks of worsening acute kidney injury (AKI). A recent retrospective cohort study of trauma-induced rib fracture patients found that a short course of NSAID use did not worsen AKI compared to controls and the authors concluded that NSAIDs may be underutilized in severely injured trauma patients. [ 45 ]
Lidocaine: Transdermal and Intravenous
The use of transdermal lidocaine has been mixed, with one study demonstrating no difference in intravenous opioid use or pain scores when comparing 5% lidocaine patch with placebo, while a second study showed significantly lower average pain scores after day 5 and significantly lower total meperidine use in patients who received 5% lidocaine patches compared to placebo [ 46 , 47 ]. Intravenous lidocaine has also been evaluated as an analgesic in rib fracture patients with more success. A single-center, double blinded randomized controlled trial compared intravenous lidocaine plus usual analgesics to placebo plus usual analgesics and found a significant reduction in pain with movement in the lidocaine group [ 47 ]. A retrospective study assessed the ability of intravenous lidocaine to reduce overall opioid use and pain scores in patients with rib fractures; they found a 30% reduction in pain scores amongst intravenous lidocaine patients, although they noted that intravenous lidocaine was less effective in patients with a history of substance abuse [ 48 ].
It has become widely accepted that subanesthetic doses of ketamine produce analgesia and can also increase the effectiveness of opioids [ 49 ]. A recent double-blinded, randomized placebo-controlled trial examined the efficacy of low dose ketamine as a primary mode of analgesia in patients with rib fractures [ 50 ]. The study found that low dose ketamine failed to affect the 24-h numeric pain scores or OME totals; however, a decrease in OME was demonstrated in patients with an ISS greater than 15.
Regional Anesthesia
Many studies have evaluated the efficacy of regional anesthesia modalities on pain reduction and morbidity outcomes, but much of the data has been conflicting. One meta-analysis and a separate systematic review of 32 randomized controlled trials demonstrated that epidural analgesia provided better pain relief than other locoregional modalities; however, there were no differences observed for secondary endpoints such as ICULOS or pulmonary complications [ 7 , 13 ]. The Eastern Association for the Surgery of Trauma and the Chest Wall Injury Society performed a systematic review and meta-analysis on analgesia strategies for older adults with multiple rib fractures and found that epidural and other regional analgesia techniques did not have any effect on pneumonia, HLOS, length of mechanical ventilation, or mortality [ 11 ]. Based on their work, they currently offer no recommendation for or against the use of epidurals or other regional anesthesia techniques in older patients with rib fractures, though multiple other professional societies continue to recommend epidural placement for older adults (> 65 years) with three or more rib fractures when there are no contraindications [ 4 , 26 , 51 ]. Interestingly, a recent large retrospective study examined the effects of timing of regional anesthesia techniques and found that early regional anesthesia (within 24 h) had a decreased incidence of unplanned intubation, ICU admission, and an increased odds of discharge to home when compared to the late regional anesthesia group (after 24 h) in elderly patients with rib fractures. [ 52 ]
Given the inconsistency in data and differing recommendations among trauma societies, we recommend the use of a regional technique in patients with rib fractures, but the choice of that preferred regional technique should be based on local expertise. We typically utilize several different regional anesthesia techniques depending on our patients’ comorbidities, contraindication profiles, and individual provider preference. Examples of regional techniques that are utilized aside from epidurals include erector spinae plane block (ESPB), paravertebral block (PVB), serratus anterior plane block (SAPB), and intercostal nerve block (ICNB) (Fig. 1 ).

An anatomical schematic and an associated series of static ultrasound images that delineate various regional anesthesia blocks that can be used for analgesia for rib fracture patients. ESPB = erector spinae plane block; PVB = paravertebral block; SAPB = serratus anterior plane block; ICNB = intercostal nerve block; T = trapezius; R = rhomboid; ES = erector spinae; LD = latissimus dorsi; SA = serratus anterior; EI = external intercostal; II = internal intercostal; IMI = innermost intercostal; TP = transverse process; PVS = paravertebral space
Neuraxial/Epidural Anesthesia
The mainstay of regional anesthesia for rib fractures has been thoracic epidural anesthesia, and it is generally our first choice at our institution [ 12 ]. However, many trauma patients have contraindications to epidural placement such as anticoagulation or coagulopathy, unstable spine with or without spinal cord injury, and/or hemodynamic instability. [ 12 , 13 , 53 ] In these cases, a newer regional technique can be chosen which can provide benefit with a lower-risk profile.
Erector Spinae Plane Block (ESPB)
The ESPB is an ultrasound-guided myofascial plane block that targets the plane between the erector spinae muscle group and a transverse process. Used as a single-shot or catheter-based technique, this procedure may allow for local anesthetic to diffuse to both dorsal and ventral rami, which can supply the ribcage. ESBP is becoming more popular given its low risk of complications (spinal cord injury, epidural hematoma, hemodynamic instability) and anticoagulation is not a contraindication according to the joint European Society of Anesthesiology and Intensive Care (ESAIC) and European Society of Regional Anesthesia (ESRA) guidelines [ 54 ]. Although some studies have demonstrated ESPB to have positive outcomes as an effective technique for analgesia in rib fractures, until there is more conclusive evidence, its use should be determined based on the risk profile of a given patient and provider comfort [ 55 , 56 , 57 ].
Paravertebral Block (PVB)
Classically a blind, surface anatomy-based technique, the PVB has more recently been adapted utilizing ultrasound-guidance to facilitate accessing the area alongside a vertebral body near where the spinal nerves emerge from the intervertebral foramen. In the thoracic region, a single-shot or catheter-based technique can allow for local anesthetic to remain localized to the level injected, or it may spread to contiguous levels, the intercostal space, and/or the epidural space, which can produce chest wall analgesia. PVB is a favorable technique for rib fractures given its fewer adverse effects, complications, and contraindications. Although retrospective studies have shown reductions in ICU admission and mortality and improved analgesia, they are an appropriate alternative regional option that can be used in certain circumstances [ 58 ].
Serratus Anterior Plane Block (SAPB)
The SAPB is an ultrasound-guided block that targets the lateral cutaneous branches of the thoracic intercostal nerves. These nerves can be blocked in either the deep or superficial potential spaces that bound the serratus anterior via single shot or catheter-based techniques. SAPB has few complications and contraindications, but there is an increased risk of pneumothorax. One notable advantage of SAPB is that it can be performed with less patient cooperation as it can be performed in the supine position with minimal repositioning. There are few quality studies assessing analgesic efficacy and outcomes in rib fracture patients receiving SAPB; however, SAPB is a reasonable technique to consider in patients who have positioning limitations [ 59 ].
Intercostal Nerve Block (ICNB)
The ICNB can be performed via a landmark-based or ultrasound-guided technique to target the anterior/ventral rami of the T1-T11 spinal nerves. Although catheters can be placed, the single-shot technique is more practical for this regional technique, especially for multiple rib fractures. ICNB is lower risks and fewer contraindications than epidurals; however, there is a higher risk of pneumothorax and vascular damage. Very limited evidence exists regarding ICNB in terms of analgesic efficacy and outcomes in rib fracture patients, though it appears to be a relatively safe technique that can offer single or multiple rib analgesia in specific patients [ 60 ].
Conclusions
Rib fractures are associated with significant morbidity and mortality and are a burden to our healthcare system. Scoring systems should be used to guide the management of patients with rib fractures as they have been shown to decrease morbidity and mortality. Enhancing pulmonary hygiene and analgesia regimens remain the therapeutic target of rib fracture management. IS should be utilized in all patients for its prognostic and therapeutic purposes, and noninvasive ventilation may decrease the rates of pneumonia. Although no specific analgesic modality has been shown to be superior to others, multimodal analgesia regimens combining systemic medications and regional anesthesia techniques should be utilized to enhance pain control.
Data availability
No datasets were generated or analysed during the current study.
Sarode AL, Ho VP, Pieracci FM, Moorman ML, Towe CW. The financial burden of rib fractures: National estimates 2007 to 2016. Injury. 2021;52(8):2180–7.
Article PubMed PubMed Central Google Scholar
Kim M, Moore JE. Chest Trauma: Current Recommendations for Rib Fractures, Pneumothorax, and Other Injuries. Curr Anesthesiol Rep. 2020;10(1):61–8.
Sharma OP, Oswanski MF, Jolly S, Lauer SK, Dressel R, Stombaugh HA. Perils of rib fractures. Am Surg. 2008;74(4):310–4.
Article PubMed Google Scholar
Tignanelli CJ, Rix A, Napolitano LM, Hemmila MR, Ma S, Kummerfeld E. Association Between Adherence to Evidence-Based Practices for Treatment of Patients With Traumatic Rib Fractures and Mortality Rates Among US Trauma Centers. JAMA Netw Open. 2020;3(3): e201316.
Witt CE, Bulger EM. Comprehensive approach to the management of the patient with multiple rib fractures: a review and introduction of a bundled rib fracture management protocol. Trauma Surg Acute Care Open. 2017;2(1): e000064.
Bulger EM, Arneson MA, Mock CN, Jurkovich GJ. Rib fractures in the elderly. J Trauma. 2000;48(6):1040–6; discussion 6–7.
Peek J, Smeeing DPJ, Hietbrink F, Houwert RM, Marsman M, de Jong MB. Comparison of analgesic interventions for traumatic rib fractures: a systematic review and meta-analysis. Eur J Trauma Emerg Surg. 2019;45(4):597–622.
Coary R, Skerritt C, Carey A, Rudd S, Shipway D. New horizons in rib fracture management in the older adult. Age Ageing. 2020;49(2):161–7.
Koushik SS, Bui A, Slinchenkova K, Badwal A, Lee C, Noss BO, et al. Analgesic Techniques for Rib Fractures-A Comprehensive Review Article. Curr Pain Headache Rep. 2023.
Elkins MR. Physiotherapy management of rib fractures. J Physiother. 2023;69(4):211–9.
.Mukherjee K, Schubl SD, Tominaga G, Cantrell S, Kim B, Haines KL, et al. Non-surgical management and analgesia strategies for older adults with multiple rib fractures: A systematic review, meta-analysis, and joint practice management guideline from the Eastern Association for the Surgery of Trauma and the Chest Wall Injury Society. J Trauma Acute Care Surg. 2023;94(3):398–407. Meta-analysis and joint practice management guideline for rib fractures.
Rogers FB, Larson NJ, Rhone A, Amaya D, Olson-Bullis BA, Blondeau BX. Comprehensive Review of Current Pain Management in Rib Fractures With Practical Guidelines for Clinicians. J Intensive Care Med. 2023;38(4):327-39.
Hammal F, Chiu C, Kung JY, Bradley N, Dillane D. Pain management for hospitalized patients with rib fractures: A systematic review of randomized clinical trials. J Clin Anesth. 2023;92:111276. Systematic review of randomized trials related to pain management for rib fractures.
Barry R, Thompson E. Outcomes after rib fractures in geriatric blunt trauma patients. Am J Surg. 2018;215(6):1020-3.
Hardin KS, Leasia KN, Haenel J, Moore EE, Burlew CC, Pieracci FM. The Sequential Clinical Assessment of Respiratory Function (SCARF) score: A dynamic pulmonary physiologic score that predicts adverse outcomes in critically ill rib fracture patients. J Trauma Acute Care Surg. 2019;87(6):1260–8.
Fokin A, Wycech J, Crawford M, Puente I. Quantification of rib fractures by different scoring systems. J Surg Res. 2018;229:1–8.
Terry SM SK. PIC Score: An innovative approach to improve outcome from chest wall injury at a level 1 trauma center. American College of Surgery Trauma Quality Improvement Program (TQuIP) Meeting. Chicago, IL. 2015.
Easter A. Management of patients with multiple rib fractures. Am J Crit Care. 2001;10(5):320–7; quiz 8–9.
Ziegler DW, Agarwal NN. The morbidity and mortality of rib fractures. J Trauma. 1994;37(6):975–9.
Article CAS PubMed Google Scholar
Maxwell CA, Mion LC, Dietrich MS. Hospitalized injured older adults: clinical utility of a rib fracture scoring system. J Trauma Nurs. 2012;19(3):168–74; quiz 75–6.
Pressley CM, Fry WR, Philp AS, Berry SD, Smith RS. Predicting outcome of patients with chest wall injury. Am J Surg. 2012;204(6):910–3; discussion 3–4.
Chen J, Jeremitsky E, Philp F, Fry W, Smith RS. A chest trauma scoring system to predict outcomes. Surgery. 2014;156(4):988–93.
Chapman BC, Herbert B, Rodil M, Salotto J, Stovall RT, Biffl W, et al. RibScore: A novel radiographic score based on fracture pattern that predicts pneumonia, respiratory failure, and tracheostomy. J Trauma Acute Care Surg. 2016;80(1):95–101.
. Bass GA, Stephen C, Forssten MP, Bailey JA, Mohseni S, Cao Y, et al. Admission Triage With Pain, Inspiratory Effort, Cough Score can Predict Critical Care Utilization and Length of Stay in Isolated Chest Wall Injury. J Surg Res. 2022;277:310–8. Retrospective cohort study to determine differences between PIC score outcomes in rib fracture patients.
Terry SM, Shoff KA, Sharrah ML. Improving Blunt Chest Wall Injury Outcomes: Introducing the PIC Score. J Trauma Nurs. 2021;28(6):386-94.
Brasel KJ, Moore EE, Albrecht RA, deMoya M, Schreiber M, Karmy-Jones R, et al. Western Trauma Association Critical Decisions in Trauma: Management of rib fractures. J Trauma Acute Care Surg. 2017;82(1):200–3.
Bergeron E, Lavoie A, Clas D, Moore L, Ratte S, Tetreault S, et al. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma. 2003;54(3):478–85.
Todd SR, McNally MM, Holcomb JB, Kozar RA, Kao LS, Gonzalez EA, et al. A multidisciplinary clinical pathway decreases rib fracture-associated infectious morbidity and mortality in high-risk trauma patients. Am J Surg. 2006;192(6):806–11.
Sum SK, Peng YC, Yin SY, Huang PF, Wang YC, Chen TP, et al. Using an incentive spirometer reduces pulmonary complications in patients with traumatic rib fractures: a randomized controlled trial. Trials. 2019;20(1):797.
Butts CA, Brady JJ 3rd, Wilhelm S, Castor L, Sherwood A, McCall A, et al. Do simple beside lung function tests predict morbidity after rib fractures? Am J Surg. 2017;213(3):473–7.
Sadler CA, Burgess JR, Dougherty KE, Collins JN. Bedside Incentive Spirometry Predicts Risk of Pulmonary Complication in Patients with Rib Fractures. Am Surg. 2019;85(9):1051–5.
Halub ME, Spilman SK, Gaunt KA, Lamb KD, Jackson JA, Oetting TW, et al. High-flow nasal cannula therapy for patients with blunt thoracic injury: A retrospective study. Can J Respir Ther. 2016;52(4):110–3.
PubMed PubMed Central Google Scholar
Hsu JM, Clark PT, Connell LE, Welfare M. Efficacy of high-flow nasal prong therapy in trauma patients with rib fractures and high-risk features for respiratory deterioration: a randomized controlled trial. Trauma Surg Acute Care Open. 2020;5(1): e000460.
Pelaez CA, Jackson JA, Hamilton MY, Omerza CR, Capella JM, Trump MW. High flow nasal cannula outside the ICU provides optimal care and maximizes hospital resources for patients with multiple rib fractures. Injury. 2022;53(9):2967–73.
Bolliger CT, Van Eeden SF. Treatment of multiple rib fractures. Randomized controlled trial comparing ventilatory with nonventilatory management. Chest. 1990;97(4):943–8.
Gunduz M, Unlugenc H, Ozalevli M, Inanoglu K, Akman H. A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg Med J. 2005;22(5):325–9.
Article CAS PubMed PubMed Central Google Scholar
Hernandez G, Fernandez R, Lopez-Reina P, Cuena R, Pedrosa A, Ortiz R, et al. Noninvasive ventilation reduces intubation in chest trauma-related hypoxemia: a randomized clinical trial. Chest. 2010;137(1):74–80.
Kourouche S, Buckley T, Munroe B, Curtis K. Development of a blunt chest injury care bundle: An integrative review. Injury. 2018;49(6):1008–23.
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–57.
Wei S, Green C, Truong VTT, Howell J, Ugarte SM, Albarado R, et al. Implementation of a multi-modal pain regimen to decrease inpatient opioid exposure after injury. Am J Surg. 2019;218(6):1122–7.
Burton SW, Riojas C, Gesin G, Smith CB, Bandy V, Sing R, et al. Multimodal analgesia reduces opioid requirements in trauma patients with rib fractures. J Trauma Acute Care Surg. 2022;92(3):588–96.
Esmailian M MR, Zamani M. Comparison of the analgesic effect of IV Acetaminophen and morphine sulfate in rib fracture; A randomized double-blind clinical trial. Emerg(Tehran). 2015;3(3):99‐102.
Antill AC, Frye SW, McMillen JC, Haynes JC, Ford BR, Bollig RW, et al. Treatment With Oral Versus Intravenous Acetaminophen in Elderly Trauma Patients With Rib Fractures: A Prospective Randomized Trial. Am Surg. 2020;86(8):926–32.
Bayouth L, Safcsak K, Cheatham ML, Smith CP, Birrer KL, Promes JT. Early intravenous ibuprofen decreases narcotic requirement and length of stay after traumatic rib fracture. Am Surg. 2013;79(11):1207–12.
Hatton GE, Bell C, Wei S, Wade CE, Kao LS, Harvin JA. Do early non-steroidal anti-inflammatory drugs for analgesia worsen acute kidney injury in critically ill trauma patients? An inverse probability of treatment weighted analysis. J Trauma Acute Care Surg. 2020;89(4):673–8.
Ingalls NK, Horton ZA, Bettendorf M, Frye I, Rodriguez C. Randomized, double-blind, placebo-controlled trial using lidocaine patch 5% in traumatic rib fractures. J Am Coll Surg. 2010;210(2):205–9.
Patton P, Vogt K, Priestap F, Parry N, Ball IM. Intravenous lidocaine for the management of traumatic rib fractures: A double-blind randomized controlled trial (INITIATE program of research). J Trauma Acute Care Surg. 2022;93(4):496–502. RCT comparing pain scores and satisfaction between intravenous lidocaine and placebo in rib fracture patients.
King S, Smith L, Harper C, Beam Z, Heidel E, Carico G, et al. Intravenous Lidocaine for Rib Fractures: Effect on Pain Control and Outcome. Am Surg. 2022;88(4):734-9.
Adriaenssens G, Vermeyen KM, Hoffmann VL, Mertens E, Adriaensen HF. Postoperative analgesia with i.v. patient-controlled morphine: effect of adding ketamine. Br J Anaesth. 1999;83(3):393–6.
Kugler NW, Carver TW, Juul J, Peppard WJ, Boyle K, Drescher KM, et al. Ketamine infusion for pain control in elderly patients with multiple rib fractures: Results of a randomized controlled trial. J Trauma Acute Care Surg. 2019;87(5):1181–8.
Bulger EM, Edwards T, Klotz P, Jurkovich GJ. Epidural analgesia improves outcome after multiple rib fractures. Surgery. 2004;136(2):426–30.
Proano-Zamudio JA, Argandykov D, Renne A, Gebran A, Ouwerkerk JJJ, Dorken-Gallastegi A, et al. Timing of regional analgesia in elderly patients with blunt chest-wall injury. Surgery. 2023;174(4):901–6. Retrospective study comparing early and late regional anesthesia in elderly rib fracture patients.
Peek J, Beks RB, Kingma BF, Marsman M, Ruurda JP, Houwert RM, et al. Epidural Analgesia for Severe Chest Trauma: An Analysis of Current Practice on the Efficacy and Safety. Crit Care Res Pract. 2019;2019:4837591.
Kietaibl S, Ferrandis R, Godier A, Llau J, Lobo C, Macfarlane AJ, et al. Regional anaesthesia in patients on antithrombotic drugs: Joint ESAIC/ESRA guidelines. Eur J Anaesthesiol. 2022;39(2):100–32.
Adhikary SD, Liu WM, Fuller E, Cruz-Eng H, Chin KJ. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: a retrospective cohort study. Anaesthesia. 2019;74(5):585–93.
Riley B, Malla U, Snels N, Mitchell A, Abi-Fares C, Basson W, et al. Erector spinae and serratus anterior blocks for the management of rib fractures: A retrospective exploratory matched study. Am J Emerg Med. 2020;38(8):1689–91.
Xu L, Basireddy S, Villanueva C, Horn A, Tsui BCH. Thoracic epidural anesthesia versus continuous erector spinae plane block for traumatic rib fracture analgesia: A retrospective cohort study. J Clin Anesth. 2021;75: 110519.
Womack J, Pearson JD, Walker IA, Stephens NM, Goodman BA. Safety, complications and clinical outcome after ultrasound-guided paravertebral catheter insertion for rib fracture analgesia: a single-centre retrospective observational study. Anaesthesia. 2019;74(5):594–601.
Bhalla PI, Solomon S, Zhang R, Witt CE, Dagal A, Joffe AM. Comparison of serratus anterior plane block with epidural and paravertebral block in critically ill trauma patients with multiple rib fractures. Trauma Surg Acute Care Open. 2021;6(1): e000621.
Uhlich R, Kerby JD, Bosarge P, Hu P. Use of continuous intercostal nerve blockade is associated with improved outcomes in patients with multiple rib fractures. Trauma Surg Acute Care Open. 2021;6(1): e000600.
Download references
Author information
Authors and affiliations.
Department of Anesthesia and Perioperative Care, Zuckerberg San Francisco General Hospital University of California, 1001 Potrero Avenue #3C38, San Francisco, CA, 94110, USA
Kaveh Hemati, Andrew T. Gray & Ashish Agrawal
You can also search for this author in PubMed Google Scholar
Contributions
K.H. wrote the manuscript text and prepared the table and figure. All authors reviewed and edited the manuscript.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Corresponding author
Correspondence to Kaveh Hemati .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Hemati, K., Gray, A.T. & Agrawal, A. A Comprehensive Review of the Non-operative Management of Traumatic Rib Fractures. Curr Anesthesiol Rep (2024). https://doi.org/10.1007/s40140-024-00645-w
Download citation
Accepted : 06 August 2024
Published : 22 August 2024
DOI : https://doi.org/10.1007/s40140-024-00645-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Rib fractures
- Risk assessment
- Non-invasive ventilation
- Incentive spirometry
- Multimodal analgesia
- Regional anesthesia
Advertisement
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
Evidence-Based Medicine: History, Review, Criticisms, and Pitfalls. Evidence-based medicine (EBM) is the use of high-quality clinical research in making decisions about the care of patients. Its formal origin dates back to the mid-nineteenth century, and since then, it has continued to evolve. The best research evidence, clinical expertise, and ...
The term evidence-based medicine (EBM) refers to the practice of caring for patients using the best available research evidence to guide clinical decision-making ( figure 1) [ 1,2 ]. The value of EBM is heightened in light of the following considerations:
Systematic reviews that summarize the available information on a topic are an important part of evidence-based health care. There are both research and non-research reasons for undertaking a literature review. It is important to systematically review the literature when one would like to justify the need for a study, to update personal ...
Explore the foundations of evidence-based medicine with JAMA's Users' Guide to the Medical Literature collection. Learn to understand and interpret clinical research!
A literature review forms the basis for high-quality medical education research and helps maximize relevance, originality, generalizability, and impact. A literature review provides context, informs methodology, maximizes innovation, avoids duplicative research, and ensures that professional standards are met.
Technological advances are transforming evidence generation in medicine; for these advances to impact public health, the clinical trial landscape must evolve and adapt to keep pace.
Evidence-based medicine (EBM) is a clinical discipline that bridges the gap between research and clinical practice. EBM is dedicated to make decision-making more objective and structured by better reflecting the evidence from researches, especially from studies on clinical epidemiology. It facilitates a transformation of clinical practice and ...
Evidence-based medicine (EBM) is at the core of current clinical practice. The philosophical origins of EBM date as far back as the mid-19th century earlier. David Sackett (1934-2015) considered as the father of EBM, described it as ' the conscientious, explicit and judicious use of current best evidence in making decisions about the care of ...
Learn how to use gray literature in systematic reviews and why it is important for evidence-based medicine. A peer-reviewed journal article by Paez et al.
A necessary skill for any doctor What causes disease, which drug is best, does this patient need surgery, and what is the prognosis? Although experience helps in answering these questions, ultimately they are best answered by evidence based medicine. But how do you assess the evidence? As a medical student, and throughout your career as a doctor, critical appraisal of published literature is ...
Evidence-based Practice in Healthcare This guide is designed to assist health care professionals and students become effective and efficient users of the medical literature.
After a brief review of the definition and process of evidence-based medicine, the primary focus of the module is to guide your practice of turning clinical questions into database searches and selecting the best available evidence from the results to critically appraise and apply.
Since evidence was described as a hierarchy, a compelling rationale for a pyramid was made. Evidence-based healthcare practitioners became familiar with this pyramid when reading the literature, applying evidence or teaching students.
This article presents guidelines for writing an evidence-based clinical review article for American Family Physician. First, the topic should be of common interest and relevance to family practice ...
On evidence-based medicine. In their Review published in The Lancet (July 22, p 415), Benjamin Djulbegovic and Gordon H Guyatt provide a comprehensive overview of the challenges evidence-based medicine (EBM) will probably face in the next 25 years. Rightly, they conclude that it is a triumph that no critic of EBM has ever suggested that ...
Evidence-based medicine (EBM) is the conscientious, explicit, and judicious use of current best evidence in making decisions regarding the care of individual patients. This concept has gained popularity recently, and its applications have been steadily expanding. Nowadays, the term "evidence-based" is used in numerous situations and conditions ...
18 Faculty skill, time for EBM teaching, and, in some cases, a perceived tension between patient-centered care and evidence-based care, are other identified barriers. 17, 19 Literature also has shown a lack of change in self-reported EBM skills during training among family medicine residents.
Secondary literature consists of interpretations and evaluations that are derived from or refer to the primary source literature. Examples include review articles (such as meta-analysis and systematic reviews) and reference works.
Level I: Evidence from a systematic review of all relevant randomized controlled trials. Level II: Evidence from a meta-analysis of all relevant randomized controlled trials. Level III: Evidence from evidence summaries developed from systematic reviews. Level IV: Evidence from guidelines developed from systematic reviews.
Evidence-Based Medicine: Literature Reviews When talking to your patients about complementary health approaches, you want to be able to answer the question: Is there any scientific evidence that this complementary product or practice works and is safe?
Reliability of the evidence to guide decision-making in the treatment of gastroesophageal reflux disease with acupuncture: protocol for an overview of systematic reviews Authors (first, second and last of 4)
Background Dietary guidelines recommend a shift to plant-based diets. Fortified soymilk, a prototypical plant protein food used in the transition to plant-based diets, usually contains added sugars to match the sweetness of cow's milk and is classified as an ultra-processed food. Whether soymilk can replace minimally processed cow's milk without the adverse cardiometabolic effects ...
As the name suggests, evidence-based medicine (EBM), is about finding evidence and using that evidence to make clinical decisions. A cornerstone of EBM is the hierarchical system of classifying evidence. This hierarchy is known as the levels of evidence. Physicians are encouraged to find the highest level of evidence to answer clinical questions.
Background: Lifestyle medicine (LM) is a contemporary scientific discipline with a multidisciplinary approach. Case Management offers a viable alternative for the care of patients with Type 2 Diabetes (T2D). This study aimed to identify the role and clinical applications of the lifestyle medicine case manager nurse (LMCMN) for T2D patients internationally and to analyze the role of specialist ...
The aim of this systematic review was to investigate the relationship between fractures of the floor of the orbit (blow outs) and their repercussions on eye movement, based on the available scientific literature. In order to obtain more reliable results, we opted for a methodology that could answer the guiding question of this research. To this end, a systematic review of the literature was ...
The aim of this scoping review was to identify and review current evidence-based practice (EBP) models and frameworks. Specifically, how EBP models and frameworks used in healthcare settings align with the original model of (1) asking the question, (2) ...
Purpose of Review This review summarizes current literature on the non-operative management of traumatic rib fractures, including risk assessment scores, respiratory therapy, and multimodal and regional analgesia. Recent Findings Rib fractures are associated with significant morbidity and mortality, especially in elderly patients. Risk assessment scores, such as the Pain Inspiration Cough (PIC ...