
- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Important Question
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- Question Bank
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- Toppers Notes
- Most Repeated Question
- Diagram Based Question
- Study Planner
- JEE Previous Year Paper
- JEE Mock Test
- JEE Crash Course
- JEE Sample Papers
- JEE Toppers Notes
- JEE Formula
- JEE Important Question
- JEE Mind Map
- JEE Integer-Numerical Type Question
- JEE Study Planner
- Important Info
- SRM-JEEE Previous Year Paper
- SRM-JEEE Mock Test
- VITEEE Previous Year Paper
- VITEEE Mock Test
- BITSAT Previous Year Paper
- BITSAT Mock Test
- Manipal Previous Year Paper
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- AP EAMCET Mock Test
- COMEDK Previous Year Paper
- COMEDK Mock Test
- GUJCET Previous Year Paper
- GUJCET Mock Test
- KCET Previous Year Paper
- KCET Mock Test
- KEAM Previous Year Paper
- KEAM Mock Test
- MHT CET Previous Year Paper
- MHT CET Mock Test
- TS EAMCET Previous Year Paper
- TS EAMCET Mock Test
- WBJEE Previous Year Paper
- WBJEE Mock Test
- AMU Previous Year Paper
- AMU Mock Test
- CUSAT Previous Year Paper
- CUSAT Mock Test
- AEEE Previous Year Paper
- AEEE Mock Test
- UPSEE Previous Year Paper
- UPSEE Mock Test
- CGPET Previous Year Paper
- BCECE Previous Year Paper
- JCECE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- Verbal Ability & Reading Comprehension
- Logical Reasoning & Data Interpretation
- CAT Mock Test
- CAT Important Question
- CAT Vocabulary
- CAT English Grammar
- MBA General Knowledge
- CAT Mind Map
- CAT Study Planner
- CMAT Mock Test
- SRCC GBO Mock Test
- SRCC GBO PYQs
- XAT Mock Test
- SNAP Mock Test
- IIFT Mock Test
- MAT Mock Test
- CUET PG Mock Test
- CUET PG PYQs
- MAH CET Mock Test
- MAH CET PYQs
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam

Thermodynamics Class 11 Notes PDF (Handwritten & Short Notes)
In Class 11 Chemistry Thermodynamics chapter has a good weightage which can help students to improve their overall score in the annual examination. Therefore, the Thermodynamics Class 11 Notes should be in priority for students who are struggling to retain their learning of Chemistry for a longer period of time. The Thermodynamics Class 11 Notes is an appropriate study resource because it compiles the entire lesson of Thermodynamics into a short yet precise document.
With the help of Thermodynamics Class 11 notes students can understand all those challenging topics of Thermodynamics in an easier way.
Thermodynamics Class 11 Notes PDF
The Selfstudys website provides the Thermodynamics Class 11 notes in a PDF so that students can easily access it. All students should have easy and free access to the Class 11 Chemistry notes so that they don’t need to search for here and there. The Thermodynamics notes can be very helpful for those students who are struggling to manage their time to re-read the chapter. However, with the help of Thermodynamics Class 11 Notes PDF a student can easily cover all the topics for many times that are mentioned in Class 11 Chemistry Thermodynamics.
How to Download the Thermodynamics Class 11 Notes?
To be able to cover all the concepts and topics of the Thermodynamics, students can download the Thermodynamics Class 11 notes from the Selfstudys website. Steps to download are-
- Open the Selfstudys website.
- Bring the arrow towards the NCERT Books & Solutions which can be seen in the navigation bar. The click will expand it further where you have to click on NCERT Notes.
- A new page will appear, select Chemistry from the list of subjects.
- Now select Thermodynamics from the list of chapters or you can search the Thermodynamics in the search bar.
Features of the Thermodynamics Class 11 Notes
Our subject experts and design team have collaborated to make the Thermodynamics Class 11 Notes so perfect that it can help students to easily grasp the information mentioned in the notes. Below, we have mentioned features of the Thermodynamics Class 11 Notes that make it unique and useful for others.
- Brief Explanations are Given: In the Thermodynamics Class 11 notes each and every topic are explained in an elaborate manner so that students can easily understand the topics.
- Device Adaptive: The Thermodynamics Class 11 notes are device adaptive as it can be downloaded from any of the devices: laptop, desktop, mobile, etc. or can be viewed online for free of cost.
- According to the Latest Syllabus: Topics and concepts included in the Thermodynamics Class 11 notes are organised according to the latest syllabus. Through this, both teachers and students can benefit a lot.
- Explained Through Diagrams: The topics and concepts in the Thermodynamics Class 11 notes are also explained through diagrams. By this explanation, Class 11 students can increase their conceptual understanding for the Chemistry chapter Thermodynamics.
- Provided in the PDF: The Class 11 Thermodynamics notes are provided in the PDF as it helps students to solve their doubts then and there.
- All Topics are Covered: In the Class 11 Chemistry notes, all topics of the chapter Thermodynamics according to the Class 11 Chemistry Syllabus. Detailed explanation of all the topics help students to easily grasp the information and score well in the final examination.
- Provided in a Simpler Language: These Class 11 Chemistry notes are provided in a simpler language. Through the simpler language, students can grasp all the topics and concepts in a progressive way.
Advantages of the Thermodynamics Class 11 Notes
Through the Thermodynamics Class 11 notes, both teachers and students can be benefited a lot. Those important advantages are:
- Forces to Pay Attention: These Class 11 Thermodynamics notes force students to pay attention throughout the preparation. By paying attention, students can improve their learning and understanding skills for the chapter Thermodynamics.
- Helps to Keep a Record: The Thermodynamics notes are considered to be a document that records everything discussed in the Thermodynamics as it can be helpful for both teachers and students. Through this students can easily understand the concepts and topics in a better way.
- Helps to Revise All Topics: With the help of Class 11 notes, students can easily revise all the topics of the chapter Thermodynamics. Through the last minute revision, students can easily remember the important facts, figures, topics and concepts.
- Improves Creativity Level: These Class 11 Thermodynamics notes can improvise creativity level of a student as in it all the topics and concepts are explained in a creative manner that force students to increase their creative thinking.
- Acts as a Study Tool: The Class 11 Chemistry Notes of the chapter Thermodynamics are considered as important study tools as it includes all the information. Through this, students can improvise their marks in Class 11 Chemistry board exam.
- Eye Catching Format: These notes in the PDF are organised in a way so that students can easily be attracted to the Thermodynamics notes. These eye-catching formats can attract more and more students to cover the topics of Thermodynamics.
How the Thermodynamics Class 11 Chemistry Notes Are Prepared?
The process of creating a well structured Thermodynamics Class 11 Chemistry Notes starts with understanding the Class 11 Chemistry Syllabus. After understanding the Syllabus experts refer to the prescribed NCERT Class 11 Chemistry textbooks to begin creating the revision notes of Thermodynamics.
What is the Importance of Thermodynamics Class 11 Notes for Class 11 Students?
It is very important for Class 11 students to refer to the Thermodynamics Class 11 notes. Because with the help of notes, students can understand all topics and concepts of Thermodynamics in a creative manner. Covering the topic and concept of Thermodynamics in a creative manner can aid students to improvise their creative skills. The creative skills will help students to answer the questions in the most effective manner.
Tips to Cover the Chapter Thermodynamics With the Help of Class 11 Notes
Those who are referring to the Class 11 Thermodynamics Notes to revise their CBSE syllabus can use the below-given tips to utilise the notes better.
- Look Through the Topics and Concepts: Before covering the chapter Thermodynamics, students need to look through the topics and concepts. It can help students to get a brief idea about facts, figures, topics, concepts, etc.
- Finish the Chapter: After getting a brief idea, students need to finish off the chapter with the help of Thermodynamics Class 11 notes. Through this, students can identify all types of topics and can study accordingly.
- Solving of Doubts: With the help of Class 11 Thermodynamics notes, students can solve all their doubts. This can help Class 11 students to remove any confusion regarding the chapter.
- Diagrams are Given: Some of the topics and concepts included in the chapter Thermodynamics are explained through diagrams. With the help of diagrams, students can also understand difficult topics in a better way.
- Practising Questions: After covering the Class 11 notes of the chapter Thermodynamics, students need to practise all kinds of questions. By practising questions, students can improve their marks for the chapter Thermodynamics.
- Acts as a Revision Tool: These Class 11 Thermodynamics notes can act as a revision tool. Revising all the topics while preparing for the chapter Thermodynamics can help students to improvise their marks.
Ways Thermodynamics Class 11 Notes Can Improve One’s Preparation
With the help of Thermodynamics Class 11 notes, students can change their preparation level. Students can easily track their skills and flaws with the help of Class 11 Chemistry notes. According to strengths and weaknesses, students can improve and change their preparation for the chapter Thermodynamics. This can help students to solve questions related to the chapter Thermodynamics.
What Are Thermodynamics Class 11 Notes and Why Should You Care?
The Thermodynamics Class 11 notes are considered to be the short summary of the chapter. With the help of a short summary, Class 11 students can smoothly understand all the topics included in the chapter Thermodynamics. Better understanding in the chapter can help students to improve their answering methodology and in maintaining the higher accuracy. Because of these reasons the students should care about Class 11 Chemistry Thermodynamics Notes.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

One Last Step...

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


M-Physics Tutorial
Thermodynamics class 11 notes physics chapter 12, introduction.
The foundation of thermodynamics is the conservation of energy and the fact that heat flows spontaneously from hot to cold body and not the other way around. The study of heat and its transformation to mechanical energy is called thermodynamics . It comes from a Greek word meaning “ Movement of Heat ”.
In this chapter, we shall study the laws of thermodynamics , various thermodynamic processes , basic theory of heat engines , refrigerators and Carnot engine .
Thermal Equilibrium
When the temperature of the mixture becomes almost stable with the surrounding there is no further exchange of energy. This state in thermodynamics is called thermal equilibrium . So we may say in thermal equilibrium, the temperatures of the two systems are equal.
Zeroth Law of Thermodynamics
Zeroth law of thermodynamics states that “ If two systems are in thermal equilibrium with a third system separately are in thermal equilibrium with each other .” Physical quantity whose value is equal for two systems in thermal equilibrium is called temperature (T).

Heat and Internal Energy
Heat is that form of energy which gets transferred between a system and its surrounding because of temperature difference between them. Heat flows from the body at a higher temperature to the body at lower temperature. The flow stops when the temperature equalises. i.e., the two bodies are then in thermal equilibrium .
(ii). Internal Energy
It is sum of the kinetic energies and potential energies of all the constituent molecules of the system. It is denoted by ‘U’. U depends only on the state of the system. It is a state variable which is independent of the path taken to arrive at that state.
Recommended Books
- NCERT Textbook For Class 11 Physics Part 1 & 2
- CBSE All In One Physics Class 11 2022-23 Edition
- Oswaal CBSE Chapterwise Question Bank Class 11 Physics Book
- Modern's abc Plus of Physics for Class-11 (Part I & II)
Read also: Kinetic Theory Class 11 Physics Notes Chapter 13
Work Done by a Gas
A container of cross sectional area A is fitted with a movable piston. Let the pressure of gas is P. Due to force applied by gas on piston, piston is displaced by Δx
Work done by gas,
`W=F.\triangle r`
`W=F \triangle x cos0`
`W=F \triangle x`
`W=PA \triangle x`
`W=P \triangle V`
First Law of Thermodynamics
The first law of thermodynamics is a particular form of the general law of conservation of energy. Suppose the amount of heat Q is supplied to a system. It is normally spent in two ways.
Partially, it is spent in increasing internal energy of system.
The remaining part of it is spent in expanding the body against the external pressure, i.e. in doing external work W.
If ΔU the change in internal energy " since energy can neither be created nor destroyed but only convert from one form to another ", we have then
Q = ΔU + W............(1)
If dQ, dU and dW are infinitesimal changes in heat, internal energy and work respectively, then equation (1) becomes
dQ = dU + dW
This equation represents the differential form of first law of thermodynamics .
Read also: Organic Chemistry Some Basic Principles and Techniques Class 11 Notes Chemistry Chapter 12
Limitations of First Law of Thermodynamics
The first law of thermodynamics plays an important role in thermodynamics as it can be applied to know how much work will be obtained by transferring a certain amount of heat energy in a given thermodynamic process. However, first law of thermodynamics suffers from the following limitations :
- First law of thermodynamics does not indicate the direction of heat transfer.
- First law of thermodynamics does not tell anything about the conditions under which heat can be transformed into work.
- The first law does not indicate as to why the whole of the heat energy cannot be continuously converted into mechanical work.
Specific Heat Capacity
Specific heat capacity of a substance is defined as the heat required to raise the temperature of unit mass through 1°C (or 1 K).
Heat capacity of a substance is given by
`S=\frac{\triangle Q}{\triangle T}`
If we divide S by mass of the substance m in kg, we get
`C=\frac{S}{m}=\frac{1}{m}\frac{\triangle Q}{\triangle T}`
here s is known as the specific heat capacity of the substance. It depends on the nature of the substance and its temperature. The unit of s is J kg –1 K –1 .
The specific heat at constant volume C v
It is defined as the amount of heat required to raise the temperature of a 1 mole of a gas through 1°C when its volume is kept constant. It is denoted by (C v ) and given by
`C_V=\left(\frac{\triangle Q}{\triangle T}\right)_V`
The specific heat at constant pressure C p
It is defined as the amount of heat required to raise the temperature of 1 mole of the gas through 1°C when its pressure is kept constant. It is denoted by (C p ) and given by
`C_P=\left(\frac{\triangle Q}{\triangle T}\right)_P`
Read also: Conceptual Questions for Class 11 Physics Chapter 12 Thermodynamics
Derivation of Mayer's Formula
From 1st law,
ΔQ = ΔU + ΔW = ΔU + PΔV
At constant volume ΔV = 0 so ΔQ = ΔU
`C_{v}=(\frac{ΔQ}{ΔT})_{v}=(\frac{ΔU}{ΔT})_{v}`
`C_{v}=\frac{ΔU}{ΔT}`
On the other hand, at constant pressure,
ΔQ = ΔU + PΔV
`C_{p}=(\frac{ΔQ}{ΔT})_{p}=(\frac{ΔU}{ΔT})_{p}+P(\frac{ΔV}{ΔT})_{p}`
Now, for a mole of an ideal gas
`\frac{ΔV}{ΔT}=\frac{R}{P}`
`C_{p}=(\frac{ΔU}{ΔT})_{p}+\frac{P\times R}{P}`
`C_{p}=C_{v}+R`
`C_{p}-C_{v}=R`
This formula is known as Mayer's Formula . All the three quantities (C p ), (C v ) and R in this equation should be expressed in the same units either in joule/mole°C or in cal/mole°C.
Thermodynamic state variables and equation of state
The parameters or variables which describe equilibrium states of the system are called state variables .
(i). Intensive Variable
These are the variables which are independent of the size. e.g., pressure, density and temperature.
(ii). Extensive Variable
These are the variables which depend on the size of the system. e.g., volume, mass, internal energy.
(iii). Equation of State
The relation between the state variables is called the equation of state .
Thermodynamic processes
Any change in the thermodynamic coordinates of a system is called a process . The following are familiar processes in the thermodynamics.
(i). Isothermal Process
When a thermodynamic system undergoes a process under the condition that its temperature remains constant, then the process is said to be isothermal process . The essential condition for an isothermal process is that the system must be contained in a perfectly conducting chamber.
For isothermal process,
from the first law of thermodynamics,
Hence, for an ideal gas all heat is converted into work in isothermal process .
(ii). Adiabatic Process
When a thermodynamic system undergoes a process under the condition that no heat comes into or goes out of the system, then the process is said to be adiabatic process . Such a process can occur when a system is perfectly insulated from the surroundings.
For adiabatic process,
(iii). Isobaric Process
If the working substance is taken in expanding chamber in which the pressure is kept constant, the process is called isobaric process . In this process the gas either expands or shrinks to maintain a constant pressure and hence a net amount of work is done by the system or on the system.
(iv). Isochoric Process
If a substance undergoes a process in which the volume remains unchanged, the process is called an isochoric process . The increase of pressure and temperature produced by the heat supplied to a working substance contained in a non-expanding chamber is an example of isochoric process.
For isochoric process,
ΔV = 0, W = PΔV, W = 0
(v). Quasi Static Process
A quasi-static process is defined as the process in which the deviation from thermodynamics equilibrium is infinitesimal and all the states through which the system passes during quasi-static process may be treated as aquarium states. Thus it may be defined as a succession of equilibrium states.
Heat engines
Any " cyclic " device by which heat is converted into mechanical work is called a heat engine . For a heat engine there are three essential requirements :
Source:- A hot body, at a fixed high temperature T 1 from which the heat can be drawn heat, is called source or hot reservoir.
Sink:- A cold body at a fixed lower temperature T 2 to which any amount of heat can be rejected, is called sink or cold reservoir.
Working Substance:- The material, which on being supplied with heat, performs mechanical work is called the working substance .
In a heat engine , the working substance takes in heat from the source, converts a part of it into external work, gives out the rest to the sink and returns to its initial state. This series of operations constitute a cycle. The work can be continuously obtained by performing the same cycle over and over again.
Suppose the working substance takes in an amount of heat Q 1 from the source, and gives out an amount Q 2 to the sink. Let W be the amount of work obtained. The net amount of heat absorbed by the substance is Q 1 - Q 2 , which has been actually converted into work. Applying the first law of thermodynamics to one complete cycle. We get
Q 1 - Q 2 = W
Thermal Efficiency
The thermal efficiency (e) of an engine is defined as the ratio of the work obtained to the heat taken in from the source, that is,
`\e=\frac W{Q_1}=\frac{Q_1-Q_2}{Q_1}`
`\e=1-\frac{Q_2}{Q_1}`
This equation indicates that the efficiency of the heat engine will be unity (efficiency 100%) when Q 2 = 0. This is, however, not possible in practice, This means that the engine cannot convert all the heat taken in from the source into work.
Refrigerators and heat pumps
(i). reversible process.
A reversible process is one which can be retraced in opposite order by slightly changing the external conditions. The working substance in the reverse process passes through all the stages as in the direct process in such a way that all changes occurring in the direct process are exactly repeated in the opposite order and inverse sense and no changes are left in any of the bodies participating in the process or in the surroundings.
For reversible process,
(ii). Irreversible Process
Those process which can not be retraced in the opposite order by reversing the controlling factors are known as irreversible processes .
Second law of thermodynamics
This has two statements. First is Kelvin-Planck statement which is based upon the performance of heat engine and second is Clausius statement which is based upon the performance of refrigerator.
Kelvin-Planck statement
This may be stated as, " It is impossible to construct a device which operating in a cycle, has a sole effect of extracting heat from a reservoir at performing an equivalent amount of work ". Thus, a single reservoir at a single temperature can not continuously transfer heat into work.
Clausius statement
This may be stated as, " It is impossible for a self-acting machines working in a cycle process, unaided by any external agency to transfer heat from a body at a lower temperature to a body at a higher temperature. " In other words it may be stated as "Heat cannot flow itself from a colder to a hotter body".
Reversible and Irreversible Processes
Reversible Process: A thermodynamic process is said to be reversible if the process can be turned back such that both the system and the surroundings return to their original states, with no other change anywhere else in the universe. Ex- extension of springs, slow adiabatic compression or expansion of gases.
Irreversible Process: An irreversible process can be defined as a process in which the system and the surroundings do not return to their original condition once the process is initiated. Ex- Relative motion with friction, Heat transfer.
Carnot Engine
A reversible heat engine operating between two temperatures is called a Carnot engine and the sequences of steps constituting one cycle is called the Carnot cycle .
Carnot Theorem
Carnot gave the most important results which are:
No engine can have efficiency more than that of the Carnot engine.
The efficiency of the Carnot engine is independent of the nature of the working substance.
Thermodynamics : It is study of exchange of heat energy among bodies and conversion of heat energy into machenical energy and vice-versa.
Thermal Equilibrium : Two or more systems are said to be in thermal equilibrium, if there is no exchange of heat energy between them when they are brought in thermal contact.
Internal Energy : It is sum of the kinetic energy of all constituent particles of the system and the potential energy of interaction among these particles.
Isothermal Process : The process during which the temperature of the system remains constant.
Adiabatic Process : The process during which heat is neither given to the system, nor taken from it.
Isochoric Process : The process during which the volume remains constant.
Isobaric Process : The process during which the pressure remains constant.
Quasi-static Process : The process in which the system departs only infinitesimally from the equilibrium state.
Cyclic Process : The process in which the system returns to its initial state.
Reversible Process : The process in which the system can be retraced to its original state by reversing the conditions.
Irreversible Process : The process in which the system cannot be retraced to its original state by reversing the conditions.
Heat Engine : It is a device by which a system is made to undergo a cyclic process that results in conversion of heat into work.
Zeroth law of thermodynamics states that ‘two systems in thermal equilibrium with a third systems are in thermal equilibrium with each other’.
The internal energy of an ioslated system is constant.
Second law of thermodynamics does not allow some processes which are consistent with the first law of thermodynamics. It states
Clausius statement : No process is possible whose sole result is the transfer of heat from a colder object to a hotter object.
Kelvin-Planck statement : No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work.

- Thermodynamics Chapter 12 Physics NCERT PDF Book
- Organic Chemistry Some Basic Principles and Techniques Class 11 Notes Chemistry Chapter 12
- Class 11 Physics All Chapter wise Notes

- Class 11th (Physics)
- Class 12th (Physics)
- Classical Mechanics
- Electronics
- Electrostatics
- Modern Physics
- Physics Quiz
- Plasma Physics
- Quantum Mechanics
- Statistical Mechanics
- Thermodynamics
- NET Physics
- Class 11 (Chemistry)
- Class 12 (Chemistry)
- B.Sc.+M.Sc. (Physics)
- B.Ed. Entrance Paper
- Dissertation
- M.Sc. Paper
- Terms of Use
Talk to our experts
1800-120-456-456
Thermodynamics Class 11 Notes: CBSE Chapter 5

Thermodynamics Class 11 Chemistry Notes PDF Download
Chapter 5, Thermodynamics in Class 11 Chemistry, gives insights into the principles governing energy changes in chemical reactions and physical processes. It introduces fundamental concepts such as heat, work, internal energy, enthalpy, and the laws of thermodynamics. Chapter 5 lays the foundation for understanding how energy transformations affect chemical reactions and help predict reaction spontaneity and equilibrium conditions. Mastery of these principles is essential for studying advanced topics in physical chemistry and engineering.

Chapter 5 Thermodynamics Class 11 Notes lets you quickly access and review the chapter content. For a comprehensive study experience, check out the Class 11 Chemistry Revision Notes FREE PDF here and refer to the CBSE Class 11 Chemistry Syllabus for detailed coverage. Vedantu's notes offer a focused, student-friendly approach, setting them apart from other resources and providing you with the best tools for success.
Access Class 11 Chemistry Chapter 5 Thermodynamics Class 11 Notes
Thermodynamics:.
The study of the flow of mass, heat and energy is the study of thermodynamics.
Thermodynamics terminology:
A notable part of the universe that is kept under observation is known as the system.
Surrounding:
The remaining part of the universe except for the system which isn’t kept under observation is known as surroundings.
In general, it can be stated as;
Universe = System + Surrounding
Types of the system:
a) Open system –
The system where the flow of both, mass and heat energy takes place.
Example: Human body.
b) Closed system –
The system where the flow of heat energy takes place but has constant mass.
Example: Pressure cooker.
c) Isolated system –
The system where none of the flow takes place.
Example : Thermos flask.
State of the system:
The state of the system can be defined and changed with respect to the changes in state variables i.e., P, V, T and n. These variables define the conditions of the system and change in any one of them, will change the state of the system.
Properties of the system:
Intensive properties –
Properties depending upon concentration and are independent of mass or the total number of particles in the system. They are pressure, refractive index, density, etc.
Extensive properties –
Properties depending upon the mass or the total number of particles in the system. They are volume, total energy, etc.
State and path function:
State function –
The function will be independent of the path followed but will depend upon the initial and final states while bringing up the changes in the system.
Example: internal energy, enthalpy, etc.
Path function –
The function will depend upon the path followed while bringing up the changes in the system.
Example: work, heat, etc.
Thermodynamic equilibrium:
The system remains in equilibrium when the state variables do not change and the below three types of equilibrium are satisfied.
Mechanical equilibrium –
The absence of mechanical motion, constant pressure and volume bring up the mechanical equilibrium.
Thermal equilibrium –
The constant heat and temperature with respect to time bring up thermal equilibrium.
Chemical equilibrium –
The rate of forward reaction equal to the rate of backward reaction brings up the chemical equilibrium.
Internal energy:
The sum total of the components of the energy influenced by the internal factors of the system is known as internal energy; often denoted by U or E.
The system under observation acts as an ideal gas system that depends only upon kinetic energy and hence, is the function of temperature as $U\propto T$. Thus, the internal energy is a state function.
Modes of energy transport:
The energy transferred due to temperature differences within the system and surroundings is known as heat (Q). When the system is heated, the kinetic energy of the molecules is being increased which then increases the internal energy.
The energy spent to overcome the external forces acting upon the system is known as work (W). When a system expands, the internal energy is reduced. Whereas, on the contraction of the system the internal energy is increased.
The first law of thermodynamics:
The first law of thermodynamics states that energy can neither be created nor destroyed.
\[\Delta U=Q+W\]
The sign conventions are given as;
Work done by the system = - W
Work done on the system = + W
Heat flows into the system = + Q
Heat flows out of the system = - Q
Reversibility:
The process can change its direction by very small i.e., infinitesimal change in the system or surrounding; retracking its original path reaching the same initial state. In a process to follow reversibility, there must not be any dissipative forces and the system must be in Quasi-Static State.
Quasi-static state –
Here, the system seems to be static at all time intervals but not actually in reality. The motion is so slow that the system seems to be in equilibrium with the surroundings.
Expansion work:
The work done due to changes in the volume of the system is known as expansion work. Note that, let it be expansion or compression, we take external pressure as the driving force.
Mathematically, it can be represented as;
\[W=-\int{{{P}_{ex}}dV}\]
For reversible processes, external pressure is considered equal to the pressure of the gas. Thus,
\[W=-\int{{{P}_{gas}}dV}\]
When a P – V graph is drawn, work done is represented as the area covered under it as shown;
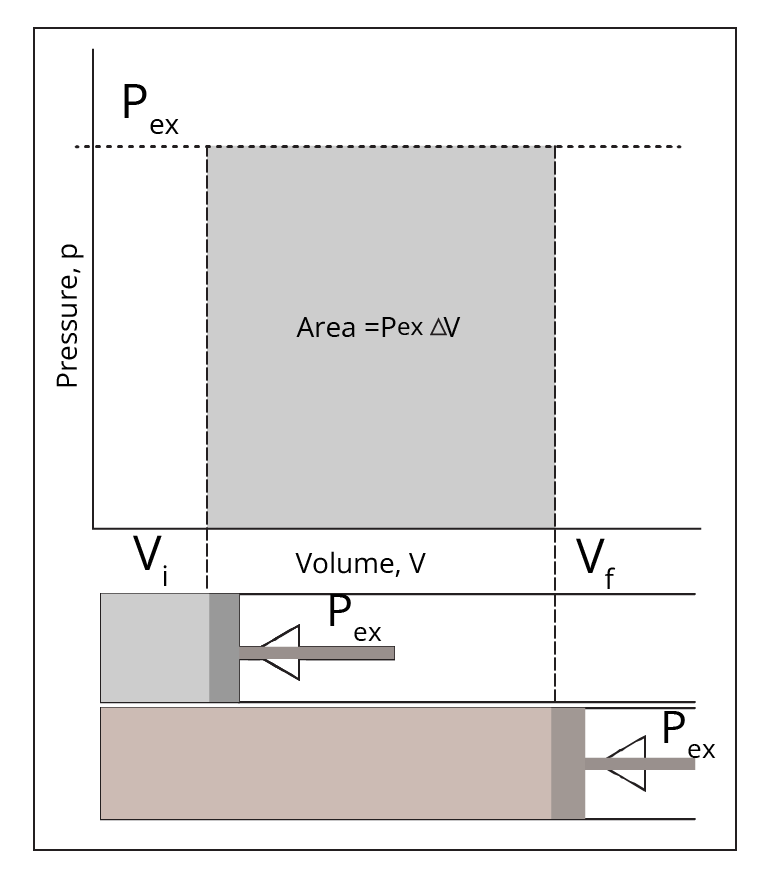
Expansion Work
Sign conventions:
Positive if the volume of the system is decreasing and negative when the volume of the system is increasing.
$\Delta U$ -
When the temperature of the system or product pressure or volume is reducing, it is negative; else is positive.
This needs to be determined by the first law of thermodynamics.
Cyclic process:
A process that comes back to its original and initial state is known as a cyclic process. A closed graph determines this process and here, $\Delta U=0$ and ${{Q}_{net}}=-{{W}_{net}}$.
A thermodynamic state function is defined as the sum of energy stored in the system and the energy used in doing work. Mathematically, can be represented as;
\[\Delta H=U+PV\]
At constant P, $\Delta H={{Q}_{P}}$.
At constant V, $\Delta U={{Q}_{V}}$.
Molar heat capacity:
At constant Pressure –
The amount of heat needed to raise the temperature of one mole of gas by a degree at constant pressure. It can be stated as;
\[{{C}_{P}}=\frac{{{Q}_{P}}}{n\Delta T}\]
At constant Volume –
The amount of heat needed to raise the temperature of one mole of gas by a degree at constant volume. It can be stated as;
\[{{C}_{V}}=\frac{{{Q}_{V}}}{n\Delta T}\]
We can now say that, $\Delta H=n{{C}_{P}}\Delta T$ and $\Delta U=n{{C}_{V}}\Delta T$ .
Types of thermodynamic processes:
Isothermal process –
The constant temperature process is known as the isothermal process. Here, $\Delta U=0$ and $\Delta H=0$ .
\[W=-2.303nRT\log \frac{{{V}_{2}}}{{{V}_{1}}}=-2.303nRT\log \frac{{{P}_{1}}}{{{P}_{2}}}\]
\[Q=2.303nRT\log \frac{{{V}_{2}}}{{{V}_{1}}}=2.303nRT\log \frac{{{P}_{1}}}{{{P}_{2}}}\]
Adiabatic process –
When the heat exchanged with the surrounding is zero, such a process is known as adiabatic process. Here,
\[T{{V}^{\gamma -1}}=C,{{T}^{\gamma }}{{P}^{1-\gamma }}=C,P{{V}^{\gamma }}=C\]
where, C is constant.
\[Q=0\Rightarrow W=\Delta U\]
\[\Delta U=n{{C}_{V}}\Delta T=\frac{\left( {{P}_{2}}{{V}_{2}}-{{P}_{1}}{{V}_{1}} \right)}{\left( \gamma -1 \right)}=\frac{\left( nR\Delta T \right)}{\left( \gamma -1 \right)}\] and
\[\Delta H=n{{C}_{P}}\Delta T\]
Isochoric process –
Constant volume process is known as isochoric process. Here, W = 0, $\Delta H=n{{C}_{P}}\Delta T$ and $\Delta U=n{{C}_{V}}\Delta T={{Q}_{V}}$.
Isobaric process –
Constant pressure process is known as isobaric process. Here, $W=-P\Delta V=-nR\Delta T$ , $\Delta H=n{{C}_{P}}\Delta T={{Q}_{P}}$ and $\Delta U=n{{C}_{V}}\Delta T$.
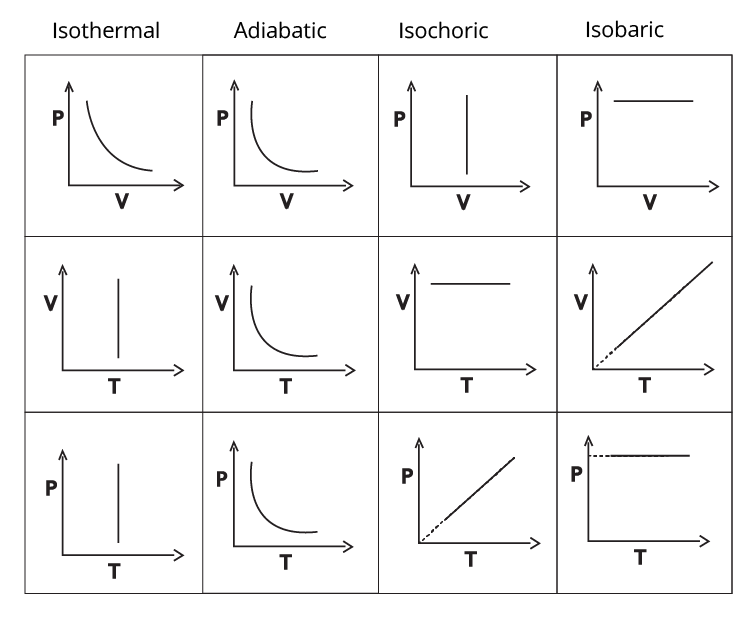
Graph of Thermodynamic processes
Note that, the P – V graphs of the isothermal and adiabatic processes are similar but the one for adiabatic is steeper than that of isothermal.
Irreversible process –
Work done is given as $W=-\int{{{P}_{ex}}dV}$ in the irreversible process. Here, we cannot say external pressure will be equal to that of the pressure of the gas.
Free expansion –
In free expansion, the external pressure of the gas is zero i.e., the gas expanding against the vacuum will have work as zero. Thus, no heat will be supplied to the process showing no changes in the temperature. Hence, it is an isothermal and adiabatic process.
Polytropic process –
A generalised form of any thermodynamic process can be represented as $P{{V}^{n}}$ = constant.
For the isothermal process, n = 1.
For adiabatic process, $n=\gamma $ .
Thermochemical equation:
A chemical equation giving you all the information like phases of reactants and products in the reaction along with energy changes associated with the same is known as a thermochemical equation.
Types of reaction:
Endothermic reaction –
The chemical reactions that absorb energy are known as endothermic reactions. Here, $\Delta H=+ve$ .
Exothermic reaction –
The chemical reactions that release energy are known as exothermic reactions. Here, $\Delta H=-ve$ .
For any chemical reaction,
\[\text{ }\!\!\Delta\!\!\text{ }{{\text{H}}_{\text{Reaction}}}\text{= }\!\!\Delta\!\!\text{ }{{\text{H}}_{\text{Products}}}\text{- }\!\!\Delta\!\!\text{ }{{\text{H}}_{\text{Reactants}}}\]
This change in enthalpy occurs due to making and breaking of bonds.
Hess law of constant heat summation:
For a reaction that takes place in a stepwise manner, the net change in enthalpy can be calculated as the enthalpy changes in each step. The governing law is known as the Hess law of constant heat summation.
Enthalpy of reactions:
Enthalpy of bond dissociation –
The energy needed to break the bonds of one-mole molecules is known as the enthalpy of bond dissociation. It is defined per mole of the molecule.
Enthalpy of combustion –
The heat released or absorbed when a mole of a substance undergoes combustion in presence of oxygen is known as enthalpy of combustion.
Enthalpy of formation –
The heat released or absorbed when a mole of a compound is formed from its constituent elements under their standard elemental forms is known as enthalpy of formation.
Enthalpy of atomization –
The energy required to convert any substance to gaseous atoms is known as the enthalpy of atomization. It is defined per mole of the gaseous atoms.
Enthalpy of sublimation –
The heat required to change a mole of a substance from solid-state to its gaseous state at STP is known as enthalpy of sublimation.
Enthalpy of phase transition –
The phase transition from one phase to another release or absorbs a particular standard enthalpy which is known as enthalpy of phase transition.
Enthalpy of ionization –
The amount of energy an isolated gaseous atom will take to lose an electron in its ground state is known as the enthalpy of ionization.
Enthalpy of the solution –
The heat released or absorbed when a mole of a compound is dissolved in excess of a solvent (mostly, water) is known as enthalpy of solution.
Enthalpy of dilution –
The enthalpy change associated with the dilution process of a component in a solution at constant pressure is known as enthalpy of dilution. It is defined as energy per unit mass or amount of substance.
The second law of thermodynamics:
The state of entropy of the entire universe, as an isolated system will always increase over time, is the standard statement of the second law of thermodynamics.
The first law of thermodynamics states the conversion of energy in a process but does not explain the feasibility of the same. This point gave rise to the need for the second law of thermodynamics.
Types of processes:
Spontaneous process –
The spontaneous process has the tendency to take place naturally and no external work is needed to carry out the same.
Non-spontaneous process –
The non-spontaneous process is driven by external work and cannot be performed naturally.
The measure of randomness or disorder in the process of a body is known as its entropy. It is a state function and is represented as S.
The spontaneous process is the process in which the total randomness of the universe tends to increase. Thus,
\[\Delta S=\frac{{{Q}_{rev}}}{T}\]
For spontaneous change, $\Delta {{S}_{Total}}=\Delta {{S}_{System}}+\Delta {{S}_{Surrounding}}>0$ .
For reversible processes where the entropy of the universe remains constant, $\Delta {{S}_{Total}}=0$.
Entropy changes in thermodynamic processes:
The entropy changes in any thermodynamic process can be mathematically represented as;
\[\Delta S=n{{C}_{V}}\ln \frac{{{T}_{2}}}{{{T}_{1}}}+nR\ln \frac{{{V}_{2}}}{{{V}_{1}}}\]
\[\Delta S=nR\ln \frac{{{V}_{2}}}{{{V}_{1}}}\]
\[\Delta S=n{{C}_{V}}\ln \frac{{{T}_{2}}}{{{T}_{1}}}\]
\[\Delta S=n{{C}_{P}}\ln \frac{{{T}_{2}}}{{{T}_{1}}}\]
\[\Delta S=0\]
Gibbs free energy:
This gives us the most convenient parameter to judge the spontaneity of the process from the perspective of the system. At constant temperature it can be represented as;
\[\Delta {{G}_{sys}}=\Delta H-T\Delta {{S}_{sys}}\]
At constant temperature and pressure, $\Delta G=-T\Delta {{S}_{Total}}$ .
For the process to be spontaneous, $\Delta G<0$.
Third law of thermodynamics:
The entropy of the system will approach a constant value as its temperature approaches absolute zero is the empirical statement of the third law of thermodynamics.
Class 11 Chemistry Chapter 5 Important Topics and Subtopics Covered
Class 11 chemistry chapters 5 details, and formulas and concepts., 1. first law of thermodynamics:.
$\Delta$ U = q + w
2. Enthalpy
$\Delta$ H = 𝐻 final −𝐻 initial
Change in enthalpy represents the heat absorbed or released at constant pressure.
2. Heat Transfer:
q = mc $\Delta$ T
3. Gibbs Free Energy
$\Delta$ G = $\Delta$ H - T $\Delta$ S
$\Delta$ S = $\frac{q_{\text{rev}}}{T}$
Change in entropy represents the dispersal of energy in a system during a reversible process.
Importance of Revision Notes for Class 11 Chemistry Chapter 5
Summarises Key Points: Condenses important concepts for quick review.
Saves Time: Provides a fast way to revise before exams.
Highlights Essentials: Focuses on crucial topics and definitions.
Improves Memory: Helps in better retention of information.
Enhances Exam Prep: Targets weak areas for more effective study.
Clarifies Concepts: Simplifies complex ideas for easier understanding.
Includes Visuals: Uses diagrams and charts for better grasp.
Boosts Confidence: Prepares students thoroughly for exams.

Tips for Learning the Class 11 Chapter 5
Focus on core processes with illustrations and examples.
Draw and label diagrams for clarity.
Create summaries of each process.
Connect concepts to everyday examples.
Solve past exam questions to test understanding.
Explain concepts to others to reinforce learning.
Revisit material frequently to retain information.
Chapter 5 Thermodynamics of Class 11 Chemistry provides a comprehensive understanding of energy changes associated with chemical reactions and physical processes. By mastering the laws of thermodynamics and concepts like enthalpy and Gibbs free energy, students are equipped to analyse reaction spontaneity and equilibrium conditions. This foundational knowledge is crucial for further studies in chemistry and related disciplines.
Related Study Materials for Class 11 Chapter 5
Revision notes links for class 11 chemistry revision notes, related study material links for class 11 chemistry, faqs on thermodynamics class 11 notes: cbse chapter 5.
1. What are Thermodynamics Class 11 notes about?
Thermodynamics Class 11 notes cover the study of energy changes in chemical reactions and physical processes, including concepts like heat, work, and internal energy.
2. What do Thermodynamics Class 11 Chemistry notes include?
Thermodynamics Class 11 Chemistry notes include the laws of thermodynamics, enthalpy, and Gibbs free energy and their applications in predicting reaction spontaneity.
3. What is covered in Class 11 Chemistry Chapter 5 notes?
Class 11 Chemistry Chapter 5 notes cover thermodynamics principles such as the laws of thermodynamics, enthalpy changes, and the concept of spontaneity.
4. Is there a Thermodynamics Class 11 notes Chemistry PDF available for download?
Yes, a Thermodynamics Class 11 notes Chemistry PDF is available for download from Vedantu.
5. What are Thermodynamics Class 11 Chemistry short notes?
Thermodynamics Class 11 Chemistry short notes provide concise summaries of key concepts, including laws of thermodynamics and enthalpy.
6. What is the focus of Thermodynamics Class 11 notes?
The focus is on understanding energy transformations, the laws of thermodynamics, and their implications for chemical reactions and equilibrium.
7. How are enthalpy changes explained in Class 11 Chemistry notes?
Enthalpy changes are explained through concepts like enthalpy of reaction, Hess’s Law, and practical measurement techniques.
8. What are the key concepts in Class 11 Chemistry Thermodynamics notes?
Key concepts include the first, second, and third laws of thermodynamics, enthalpy, entropy, and Gibbs free energy. Students can visit the Vedantu website for the Thermodynamics class 11 notes chemistry pdf download.
9. How can I get the Thermodynamics Class 11 Chemistry notes PDF?
Thermodynamics Class 11 Chemistry notes PDF can be downloaded from Vedantu that offer resources for Class 11 Chemistry.

CBSE Study Materials for Class 11
Thermodynamics
Thermodynamics deals with the concepts of heat and temperature and the inter-conversion of heat and other forms of energy. The four laws of thermodynamics govern the behaviour of these quantities and provide a quantitative description. William Thomson, in 1749, coined the term thermodynamics.
What is Thermodynamics?
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.

The video is a rapid revision of thermodynamics for JEE Main, presented by Rakhi Ma’am through short notes and previous year questions (PYQs).

Distinction Between Mechanics and Thermodynamics
The distinction between mechanics and thermodynamics is worth noting. In mechanics, we solely concentrate on the motion of particles or bodies under the action of forces and torques. On the other hand, thermodynamics is not concerned with the motion of the system as a whole. It is only concerned with the internal macroscopic state of the body.
Thermodynamics Timeline

Different Branches of Thermodynamics
Thermodynamics is classified into the following four branches:
Classical Thermodynamics
Statistical thermodynamics, chemical thermodynamics, equilibrium thermodynamics.
In classical thermodynamics, the behaviour of matter is analysed with a macroscopic approach. Units such as temperature and pressure are taken into consideration, which helps the individuals calculate other properties and predict the characteristics of the matter undergoing the process.
In statistical thermodynamics, every molecule is under the spotlight, i.e. the properties of every molecule and how they interact are taken into consideration to characterise the behaviour of a group of molecules.
Chemical thermodynamics is the study of how work and heat relate to each other in chemical reactions and in changes of states.
Equilibrium thermodynamics is the study of transformations of energy and matter as they approach the state of equilibrium.
Basic Concepts of Thermodynamics – Thermodynamic Terms
Thermodynamics has its own unique vocabulary associated with it. A good understanding of the basic concepts forms a sound understanding of various topics discussed in thermodynamics preventing possible misunderstandings.
Thermodynamic Systems

A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable. There are three types of systems:
- Isolated System – An isolated system cannot exchange energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerator, compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Surrounding
Everything outside the system that has a direct influence on the behaviour of the system is known as a surrounding.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic processes that have their unique properties, and they are:
- Adiabatic Process – A process where no heat transfer into or out of the system occurs.
- Isochoric Process – A process where no change in volume occurs and the system does no work.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Read More: Thermodynamic Process
A thermodynamic cycle is a process or a combination of processes conducted such that the initial and final states of the system are the same. A thermodynamic cycle is also known as cyclic operation or cyclic processes.
Thermodynamic Equilibrium
At a given state, all properties of a system have fixed values. Thus, if the value of even one property changes, the system’s state changes to a different one. In a system that is in equilibrium, no changes in the value of properties occur when it is isolated from its surroundings.
- When the temperature is the same throughout the entire system, we consider the system to be in thermal equilibrium .
- When there is no change in pressure at any point of the system, we consider the system to be in mechanical equilibrium .
- When the chemical composition of a system does not vary with time, we consider the system to be in chemical equilibrium .
- Phase equilibrium in a two-phase system is when the mass of each phase reaches an equilibrium level.
A thermodynamic system is said to be in thermodynamic equilibrium if it is in chemical equilibrium, mechanical equilibrium and thermal equilibrium and the relevant parameters cease to vary with time.
You may also want to check out these topics given below!
- Kelvin Planck Statement
- Darcy Weisbach Equation Derivation
- Kinetic Theory Of Gases Derivation
- Relation Between Kp And Kc
Thermodynamic Properties
Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system’s state. Thermodynamic properties may be extensive or intensive .
- Intensive properties are properties that do not depend on the quantity of matter. Pressure and temperature are intensive properties.
- In the case of extensive properties, their values depends on the mass of the system. Volume, energy, and enthalpy are extensive properties.
What is Enthalpy?
Enthalpy is the measurement of energy in a thermodynamic system. The quantity of enthalpy equals the total heat content of a system, equivalent to the system’s internal energy plus the product of volume and pressure.
Mathematically, the enthalpy, H, equals the sum of the internal energy, E, and the product of the pressure, P, and volume, V, of the system.
What is Entropy?
Entropy is a thermodynamic quantity whose value depends on the physical state or condition of a system. In other words, it is a thermodynamic function used to measure the randomness or disorder.
For example, the entropy of a solid, where the particles are not free to move, is less than the entropy of a gas, where the particles will fill the container.
Thermodynamic Potentials
Thermodynamic potentials are quantitative measures of the stored energy in a system. Potentials measure the energy changes in a system as they evolve from the initial state to the final state. Different potentials are used based on the system constraints, such as temperature and pressure.
Different forms of thermodynamic potentials along with their formula are tabulated below:
Thermodynamics Solved Problems
Calculate ΔG at 290 K for the following reaction: \(\begin{array}{l}2NO_{(g)} + O_{2(g)} + 2NO_{2(g)} \end{array} \)
ΔH = -120kJ and ΔS = -150JK -1
To make the unit of ΔS the same as ΔH, we have to convert the unit of ΔS as follows:
We know that,
Therefore, ΔG is -77kJ.
Watch the video to know the top seven JEE Thermodynamics questions.

Laws of Thermodynamics
Thermodynamics laws define the fundamental physical quantities like energy, temperature and entropy that characterize thermodynamic systems at thermal equilibrium. These thermodynamics laws represent how these quantities behave under various circumstances.
How many laws of thermodynamics are there?
There are four laws of thermodynamics and are given below:
- Zeroth law of thermodynamics
- First law of thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
In the next few sections, we will discuss each of the laws of thermodynamics in detail.
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
This means that if system A is in thermal equilibrium with system C and system B is also in equilibrium with system C, then system A and B are also in thermal equilibrium.
An example demonstrating the Zeroth Law
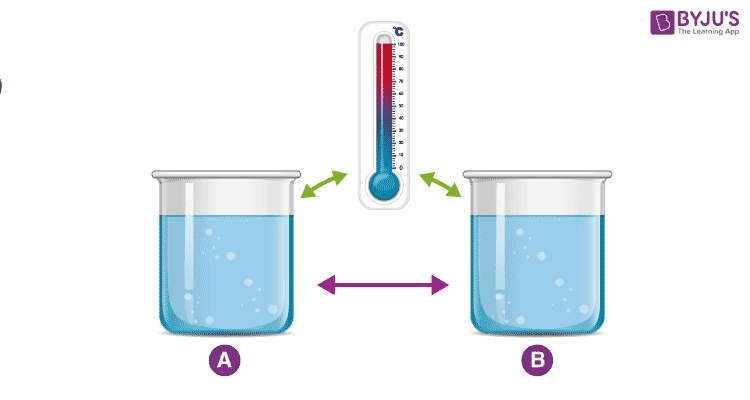
Consider two cups A and B, with boiling water. When a thermometer is placed in cup A, it gets warmed up by the water until it reads 100 °C. When it reads 100 °C, we say that the thermometer is in equilibrium with cup A. When we move the thermometer to cup B to read the temperature, it continues to read 100 °C. The thermometer is also in equilibrium with cup B. By keeping in mind the zeroth law of thermodynamics, we can conclude that cup A and cup B are in equilibrium with each other.
The zeroth law of thermodynamics enables us to use thermometers to compare the temperature of any two objects that we like.
First Law of Thermodynamics
First law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed, but it can be changed from one form to another.
The first law of thermodynamics may seem abstract, but we will get a clearer idea if we look at a few examples of the first law of thermodynamics.
First Law Of Thermodynamics Examples:
- Plants convert the radiant energy of sunlight to chemical energy through photosynthesis. We eat plants and convert the chemical energy into kinetic energy while we swim, walk, breathe, and scroll through this page.
- Switching on light may seem to produce energy, but it is electrical energy that is converted.
Read More: First Law of Thermodynamics
Second Law of Thermodynamics
Second law of thermodynamics states that the entropy in an isolated system always increases. Any isolated system spontaneously evolves towards thermal equilibrium—the state of maximum entropy of the system.
The entropy of the universe only increases and never decreases. Many individuals take this statement lightly and for granted, but it has an extensive impact and consequence.
Visualizing the second law of thermodynamics
If a room is not tidied or cleaned, it invariably becomes more messy and disorderly with time. When the room is cleaned, its entropy decreases, but the effort to clean it has resulted in increased entropy outside the room exceeding the entropy lost.
Read More: Second Law of Thermodynamics
The video below dives deep into the second law of thermodynamics and will help one take a closer look at how entropy explains disorderliness.

Third Law of Thermodynamics
Third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.
The entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy.
Third Law Of Thermodynamics Examples:
Let us consider steam as an example to understand the third law of thermodynamics step by step:
- The molecules within it move freely and have high entropy.
- If one decreases the temperature below 100 °C, the steam gets converted to water, where the movement of molecules is restricted, decreasing the entropy of water.
- When water is further cooled below 0 °C, it gets converted to solid ice. In this state, the movement of molecules is further restricted and the entropy of the system reduces more.
- As the temperature of the ice further reduces, the movement of the molecules in them is restricted further and the entropy of the substance goes on decreasing.
- When the ice is cooled to absolute zero, ideally, the entropy should be zero. But in reality, it is impossible to cool any substance to zero.
Read More: Third Law of Thermodynamics
Thermodynamics Examples in Daily Life
Whether we are sitting in an air-conditioned room or travelling in any vehicle, the application of thermodynamics is everywhere. We have listed a few of these applications below:
- Different types of vehicles such as planes, trucks and ships work on the basis of the 2nd law of thermodynamics.
- The three modes of heat transfer work on the basis of thermodynamics. The heat transfer concepts are widely used in radiators, heaters and coolers.
- Thermodynamics is involved in the study of different types of power plants such as nuclear power plants, thermal power plants.
Thermodynamics – Summary and Overview
→ In simple terms, thermodynamics deals with the transfer of energy from one form to another . → The laws of thermodynamics are:
- First law of thermodynamics: Energy can neither be created nor be destroyed, it can only be transferred from one form to another.
- Second law of thermodynamics: The entropy of any isolated system always increases.
- Third law of thermodynamics: The entropy of a system approaches a constant value as the temperature approaches absolute zero.
- Zeroth law of thermodynamics: If two thermodynamic systems are in thermal equilibrium with a third system separately, then they are in thermal equilibrium with each other.
→ Entropy is the measure of the number of possible arrangements the atoms in a system can have. → Enthalpy is the measurement of energy in a thermodynamic system.
Frequently Asked Questions – FAQs
What is the importance of the laws of thermodynamics, what is an example of negative work, can energy be destroyed or lost, fans convert electrical energy into mechanical energy – this is explained by which law, does the human body obey the laws of thermodynamics.
Stay tuned to BYJU’S and Fall in Love with Learning !

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
Very good explanation
Nicely explained with good videos and examples.
The videos on thermodynamics is very helpful. Thankyou
good explaination sir
Good explanation
Beneficial notes☺
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Class 11 Chemistry Assignments
We have provided below free printable Class 11 Chemistry Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 11 Chemistry . These Assignments for Grade 11 Chemistry cover all important topics which can come in your standard 11 tests and examinations. Free printable Assignments for CBSE Class 11 Chemistry , school and class assignments, and practice test papers have been designed by our highly experienced class 11 faculty. You can free download CBSE NCERT printable Assignments for Chemistry Class 11 with solutions and answers. All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Chemistry Class 11. Students can click on the links below and download all Pdf Assignments for Chemistry class 11 for free. All latest Kendriya Vidyalaya Class 11 Chemistry Assignments with Answers and test papers are given below.
Chemistry Class 11 Assignments Pdf Download
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 11 Chemistry . Students and teachers can download and save all free Chemistry assignments in Pdf for grade 11th. Our expert faculty have covered Class 11 important questions and answers for Chemistry as per the latest syllabus for the current academic year. All test papers and question banks for Class 11 Chemistry and CBSE Assignments for Chemistry Class 11 will be really helpful for standard 11th students to prepare for the class tests and school examinations. Class 11th students can easily free download in Pdf all printable practice worksheets given below.
Topicwise Assignments for Class 11 Chemistry Download in Pdf
Advantages of Class 11 Chemistry Assignments
- As we have the best and largest collection of Chemistry assignments for Grade 11, you will be able to easily get full list of solved important questions which can come in your examinations.
- Students will be able to go through all important and critical topics given in your CBSE Chemistry textbooks for Class 11 .
- All Chemistry assignments for Class 11 have been designed with answers. Students should solve them yourself and then compare with the solutions provided by us.
- Class 11 Students studying in per CBSE, NCERT and KVS schools will be able to free download all Chemistry chapter wise worksheets and assignments for free in Pdf
- Class 11 Chemistry question bank will help to improve subject understanding which will help to get better rank in exams
Frequently Asked Questions by Class 11 Chemistry students
At https://www.cbsencertsolutions.com, we have provided the biggest database of free assignments for Chemistry Class 11 which you can download in Pdf
We provide here Standard 11 Chemistry chapter-wise assignments which can be easily downloaded in Pdf format for free.
You can click on the links above and get assignments for Chemistry in Grade 11, all topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here topic-wise Chemistry Grade 11 question banks, revision notes and questions for all difficult topics, and other study material.
We have provided the best collection of question bank and practice tests for Class 11 for all subjects. You can download them all and use them offline without the internet.
Related Posts

Class 11 Mathematics Probability Assignments

Class 11 Engineering Graphics Assignments

Class 11 Home Science Assignments
myCBSEguide
- Entrance Exam
- Competitive Exams
- ICSE & ISC
- Teacher Exams
- UP Board
- Uttarakhand Board
- Bihar Board
- Chhattisgarh Board
- Haryana Board
- Jharkhand Board
- MP Board
- Rajasthan Board
- Courses
- Test Generator
- Homework Help
- News & Updates

- Dashboard
- Mobile App (Android)
- Browse Courses
- New & Updates
- Join Us
- Login
- Register
No products in the cart.
Chemical Thermodynamics
Concepts of System and types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions. First law of thermodynamics -internal energy and enthalpy, heat capacity and specific heat, Hess's law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution and dilution. Second law of Thermodynamics (brief introduction) Introduction of entropy as a state function, Gibb's energy change for spontaneous and non spontaneous processes, criteria for equilibrium. Third law of thermodynamics (brief introduction).
- Test Generator
Create papers online. it's FREE .

Trusted by 1 Crore+ Students
- Student Subscription
- Student Dashboard
- Install myCBSEguide App
CBSE Sample Papers
- Test Paperss

Download myCBSEguide App
All courses.
- Entrance Exams
- Competative Exams
- Teachers Exams
- Uttrakand Board
- Bihar Board
- Chhattisgarh Board
- Haryana Board
- Jharkhand Board
- Rajasthan Board
Other Websites
- Examin8.com
CBSE Courses
- CBSE Class 12
- CBSE Class 11
- CBSE Class 10
- CBSE Class 09
- CBSE Class 08
- CBSE Class 07
- CBSE Class 06
- CBSE Class 05
- CBSE Class 04
- CBSE Class 03
- CBSE Class 02
- CBSE Class 01
- CBSE Test Papers
- CBSE MCQ Tests
- CBSE 10 Year Papers
- CBSE Syllabus
NCERT Solutions
- CBSE Revision Notes
- Terms of Service
- Privacy Policy
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT Solutions for Class 10
- NCERT Solutions for Class 09
- NCERT Solutions for Class 08
- NCERT Solutions for Class 07
- NCERT Solutions for Class 06
- NCERT Solutions for Class 05
- NCERT Solutions for Class 04
- NCERT Solutions for Class 03
- CBSE Class 12 Sample Papers
- CBSE Class 11 Sample Papers
- CBSE Class 10 Sample Papers
- CBSE Class 09 Sample Papers
- CBSE Results | CBSE Datesheet
Please Wait..

IMAGES
VIDEO
COMMENTS
6.1.1 The System and the Surroundings. A system in thermodynamics refers to that part of universe in which observations are made and remaining universe constitutes the surroundings. The surroundings include everything other than the system. System and the surroundings together constitute the universe .
Answer: The change may be represented as: Question 11. Enthalpy of combustion of carbon to carbon dioxide is - 393.5 J mol-1 .Calculate the heat released upon formation of 35.2 g of C02 from carbon and oxygen gas. Answer: The combustion equation is: C (s) + 0 2 (g) —-> C0 2 (g); AcH = - 393.5 KJ mol -1.
NCERT Solutions Class 11 Chemistry Chapter 6 - Free PDF Download *According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 5. NCERT Solutions for Class 11 Chemistry Chapter 6 Thermodynamics is provided on this page. Here, students can access detailed and explanative solutions according to the latest CBSE Syllabus 2023-24 for all the questions present in the NCERT ...
Thermodynamics Class 11 Notes Physics Chapter 12. • The branch of physics which deals with the study of transformation of heat into other forms of energy and vice-versa is called thermodynamics. Thermodynamics is a macroscopic science. It deals with bulk systems and does not go into the. molecular constitution of matter. such that it has a ...
Provided in the PDF: The Class 11 Thermodynamics notes are provided in the PDF as it helps students to solve their doubts then and there. All Topics are Covered: In the Class 11 Chemistry notes, all topics of the chapter Thermodynamics according to the Class 11 Chemistry Syllabus. Detailed explanation of all the topics help students to easily ...
Class 11 Chemistry MCQ - Thermodynamics. This set of Class 11 Chemistry Chapter 6 Multiple Choice Questions & Answers (MCQs) focuses on "Thermodynamics". These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation. 1.
It is defined as the amount of heat required to raise the temperature of 1 mole of the gas through 1°C when its pressure is kept constant. It is denoted by (Cp) and given by. CP = ( Q T)P C P = ( Q T) P. Read also: Conceptual Questions for Class 11 Physics Chapter 12 Thermodynamics.
Solution. Question 11. Enthalpy of combustion of carbon to CO 2 is -393.5 kJ mol -1. Calculate the heat released upon formation of 35.2 g of CO 2 from carbon and dioxygen gas. Solution. C + O 2 → CO 2; ΔT = -393.5 kJ. ∵ When 44 g of COz is formed from carbon and dioxygen gas, heat released = 393.5 kj.
NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics. Topics and Subtopics in NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics: QUESTIONS FROM TEXTBOOK. Question 12. 1 A geyser heats water flowing at the rate of 3.0 litres per minute from 2 7°C to 77°C.
Chapter 5 Thermodynamics Class 11 Notes lets you quickly access and review the chapter content. For a comprehensive study experience, check out the Class 11 Chemistry Revision Notes FREE PDF here and refer to the CBSE Class 11 Chemistry Syllabus for detailed coverage. Vedantu's notes offer a focused, student-friendly approach, setting them apart from other resources and providing you with the ...
Physics Notes Class 11 CHAPTER 12THERMODYNAMICSThe branch dealing with measurement of temperature is called thremometry and the devices use. easure temperature are called thermometers.HeatHeat is a form of energy called thermal energy which flows from a higher temperature body to a lower.
Assignment of Chemistry Class 11, Chemistry Thermodynamics - Study Material. Page 2 : (i)Open system, (ii)Closed system, (iii)Isolated system., , 7.What will happen to internal energy if work is done by the system?, Ans.The internal energy of the system will decrease if work is done by the system., , 8.From thermodynamic point of view, to which system the animals and plants belong?, Ans. Open ...
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter. To be specific, it explains how thermal energy is converted to or from other forms of energy and how matter is affected by this process. Thermal energy is the energy that comes from heat.
A new thermodynamic function, the Gibbs energy or Gibbs function G, can be defined as G = H-TS. ΔG = ΔH - TΔS. Gibbs energy change = enthalpy change - temperature x entropy change ΔG gives a criteria for spontaneity at constant pressure and temperature, (i) If ΔG is negative (< 0) the process is spontaneous.
Class 11 Assignments. We have provided below free printable Class 11 Chemistry Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 11 Chemistry. These Assignments for Grade 11 Chemistry cover all important topics which can come in your standard 11 tests and examinations.
Download myCBSEguide App. Test papers and course material for CBSE, Class 11, Chemistry, Chemical Thermodynamics are placed here. This course material is arranged subject-wise and topic-wise. Visitors can download these test papers and course material for free of cost.